 Found 123 hits Enz. Inhib. hit(s) with Target = 'Phospholipase A2 group V'
Found 123 hits Enz. Inhib. hit(s) with Target = 'Phospholipase A2 group V' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50601469
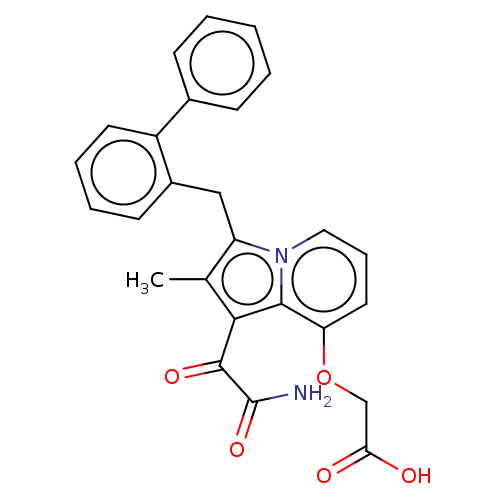
(CHEMBL5172164)Show SMILES Cc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01808
BindingDB Entry DOI: 10.7270/Q2SF316G |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50601469

(CHEMBL5172164)Show SMILES Cc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01808
BindingDB Entry DOI: 10.7270/Q2SF316G |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50263002
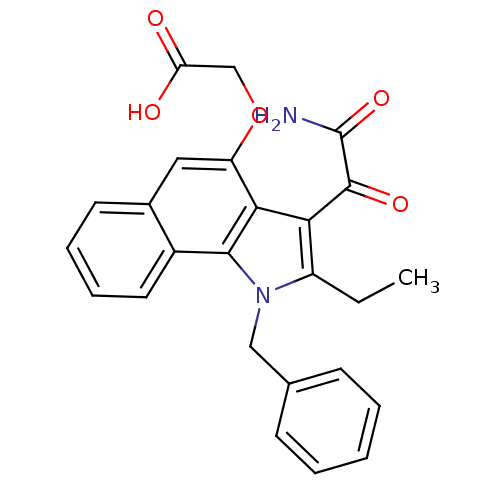
((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50263002

((2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-6,7-benz...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C25H22N2O5/c1-2-18-21(24(30)25(26)31)22-19(32-14-20(28)29)12-16-10-6-7-11-17(16)23(22)27(18)13-15-8-4-3-5-9-15/h3-12H,2,13-14H2,1H3,(H2,26,31)(H,28,29) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50055391

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-1H-ind...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OC(C)C(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-19(20(25)21(23)26)18-16(24(15)12-14-8-5-4-6-9-14)10-7-11-17(18)29-13(2)22(27)28/h4-11,13H,3,12H2,1-2H3,(H2,23,26)(H,27,28) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50262842
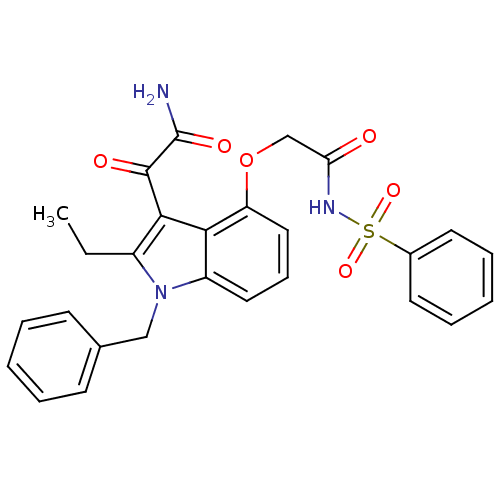
(Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl)-1-benzy...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C27H25N3O6S/c1-2-20-25(26(32)27(28)33)24-21(30(20)16-18-10-5-3-6-11-18)14-9-15-22(24)36-17-23(31)29-37(34,35)19-12-7-4-8-13-19/h3-15H,2,16-17H2,1H3,(H2,28,33)(H,29,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50263000
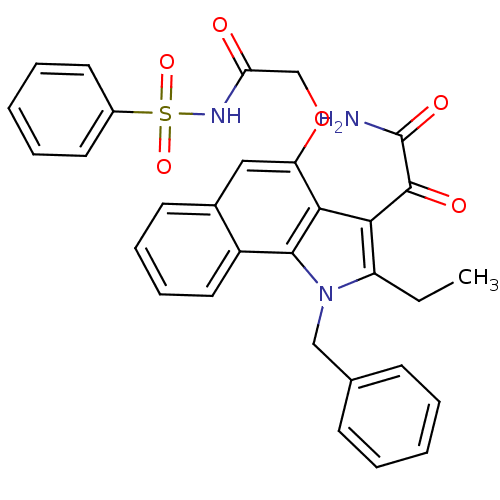
(2-(1-benzyl-2-ethyl-1H-6,7-benzoindol-4-yloxy)-N-(...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C31H27N3O6S/c1-2-24-27(30(36)31(32)37)28-25(40-19-26(35)33-41(38,39)22-14-7-4-8-15-22)17-21-13-9-10-16-23(21)29(28)34(24)18-20-11-5-3-6-12-20/h3-17H,2,18-19H2,1H3,(H2,32,37)(H,33,35) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50262842

(Benzenesulfonyl-2-(3-(2-amino-2-oxoacetyl)-1-benzy...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C27H25N3O6S/c1-2-20-25(26(32)27(28)33)24-21(30(20)16-18-10-5-3-6-11-18)14-9-15-22(24)36-17-23(31)29-37(34,35)19-12-7-4-8-13-19/h3-15H,2,16-17H2,1H3,(H2,28,33)(H,29,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50053137

((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-ethyl-indol...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C27H24N2O5/c1-2-19-21(15-18-11-6-7-12-20(18)17-9-4-3-5-10-17)29-14-8-13-22(34-16-23(30)31)25(29)24(19)26(32)27(28)33/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50274336

(1-{3-Dodecanoyl-2,4,6-trihydroxy-5-[7-hydroxy-2-(4...)Show SMILES CCCCCCCCCCCC(=O)c1c(O)c(C2CC(Oc3cc(O)ccc23)c2ccc(O)cc2)c(O)c(C(=O)CCCCCCCCCCC)c1O Show InChI InChI=1S/C45H62O8/c1-3-5-7-9-11-13-15-17-19-21-36(48)41-43(50)40(44(51)42(45(41)52)37(49)22-20-18-16-14-12-10-8-6-4-2)35-30-38(31-23-25-32(46)26-24-31)53-39-29-33(47)27-28-34(35)39/h23-29,35,38,46-47,50-52H,3-22,30H2,1-2H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2 group 5 |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50055366
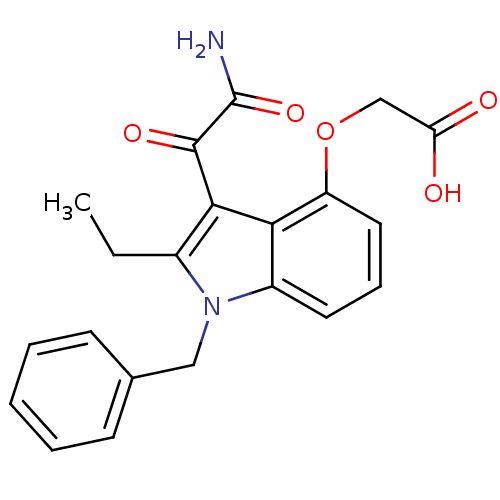
((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human sPLA2-5 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate p... |
ACS Med Chem Lett 9: 594-599 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00505
BindingDB Entry DOI: 10.7270/Q2Z60RMJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50263000

(2-(1-benzyl-2-ethyl-1H-6,7-benzoindol-4-yloxy)-N-(...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(=O)(=O)c3ccccc3)cc3ccccc3c2n1Cc1ccccc1 Show InChI InChI=1S/C31H27N3O6S/c1-2-24-27(30(36)31(32)37)28-25(40-19-26(35)33-41(38,39)22-14-7-4-8-15-22)17-21-13-9-10-16-23(21)29(28)34(24)18-20-11-5-3-6-12-20/h3-17H,2,18-19H2,1H3,(H2,32,37)(H,33,35) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50053137

((1-Aminooxalyl-3-biphenyl-2-ylmethyl-2-ethyl-indol...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OCC(O)=O)c2c1C(=O)C(N)=O Show InChI InChI=1S/C27H24N2O5/c1-2-19-21(15-18-11-6-7-12-20(18)17-9-4-3-5-10-17)29-14-8-13-22(34-16-23(30)31)25(29)24(19)26(32)27(28)33/h3-14H,2,15-16H2,1H3,(H2,28,33)(H,30,31) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50458608
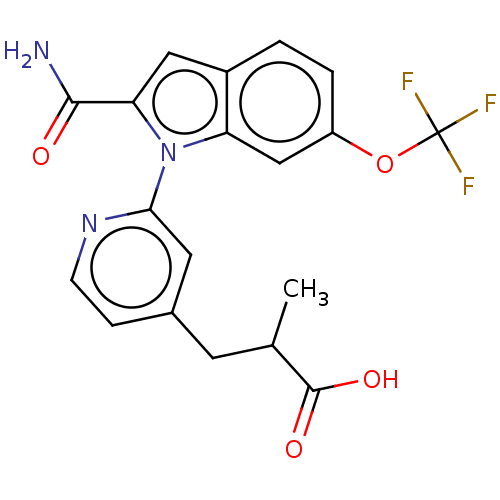
(CHEMBL4205008)Show SMILES CC(Cc1ccnc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C19H16F3N3O4/c1-10(18(27)28)6-11-4-5-24-16(7-11)25-14-9-13(29-19(20,21)22)3-2-12(14)8-15(25)17(23)26/h2-5,7-10H,6H2,1H3,(H2,23,26)(H,27,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50458608

(CHEMBL4205008)Show SMILES CC(Cc1ccnc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C19H16F3N3O4/c1-10(18(27)28)6-11-4-5-24-16(7-11)25-14-9-13(29-19(20,21)22)3-2-12(14)8-15(25)17(23)26/h2-5,7-10H,6H2,1H3,(H2,23,26)(H,27,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50055371
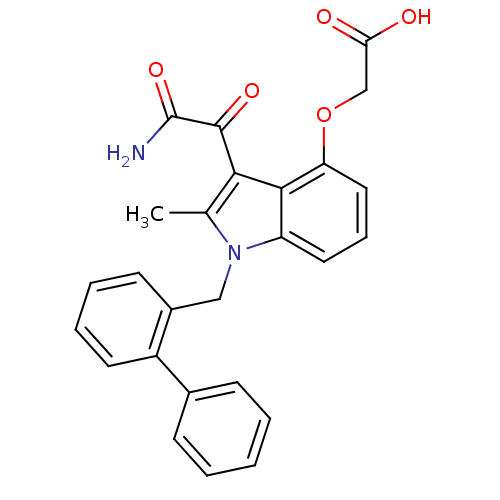
((3-aminooxalyl-1-biphenyl-2-ylmethyl-2-methyl-1H-i...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1-c1ccccc1 Show InChI InChI=1S/C26H22N2O5/c1-16-23(25(31)26(27)32)24-20(12-7-13-21(24)33-15-22(29)30)28(16)14-18-10-5-6-11-19(18)17-8-3-2-4-9-17/h2-13H,14-15H2,1H3,(H2,27,32)(H,29,30) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50333788
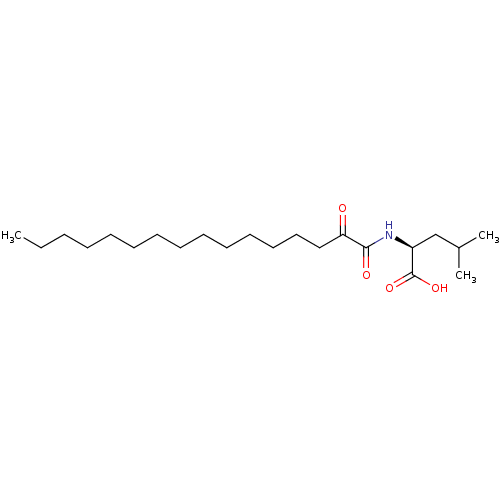
((S)-4-Methyl-2-(2-oxohexadecanamido)pentanoic acid...)Show SMILES CCCCCCCCCCCCCCC(=O)C(=O)N[C@@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C22H41NO4/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-20(24)21(25)23-19(22(26)27)17-18(2)3/h18-19H,4-17H2,1-3H3,(H,23,25)(H,26,27)/t19-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of human group 5 sPLA2 by fluorescence assay |
Bioorg Med Chem 19: 735-43 (2011)
Article DOI: 10.1016/j.bmc.2010.12.030
BindingDB Entry DOI: 10.7270/Q2JM29VG |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G5 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50186585
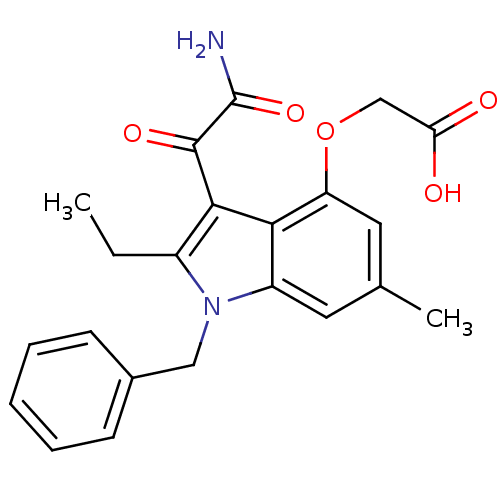
(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-6-meth...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-20(21(27)22(23)28)19-16(24(15)11-14-7-5-4-6-8-14)9-13(2)10-17(19)29-12-18(25)26/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G5 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50458612
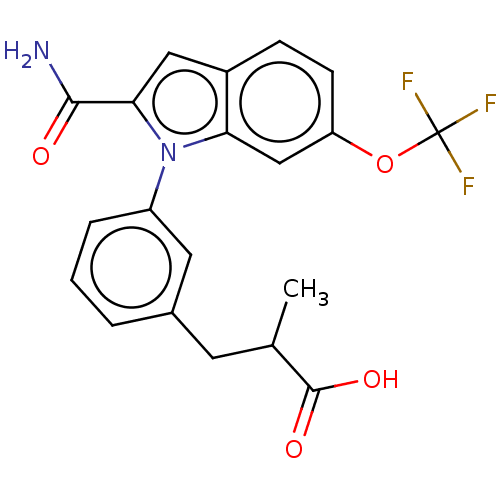
(CHEMBL4214052)Show SMILES CC(Cc1cccc(c1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C20H17F3N2O4/c1-11(19(27)28)7-12-3-2-4-14(8-12)25-16-10-15(29-20(21,22)23)6-5-13(16)9-17(25)18(24)26/h2-6,8-11H,7H2,1H3,(H2,24,26)(H,27,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50458606
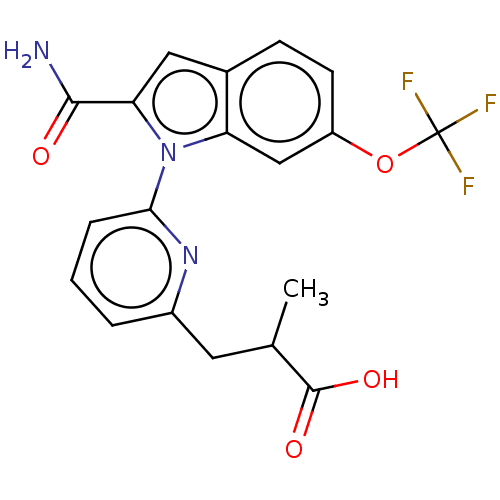
(CHEMBL4205511)Show SMILES CC(Cc1cccc(n1)-n1c(cc2ccc(OC(F)(F)F)cc12)C(N)=O)C(O)=O Show InChI InChI=1S/C19H16F3N3O4/c1-10(18(27)28)7-12-3-2-4-16(24-12)25-14-9-13(29-19(20,21)22)6-5-11(14)8-15(25)17(23)26/h2-6,8-10H,7H2,1H3,(H2,23,26)(H,27,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50274336

(1-{3-Dodecanoyl-2,4,6-trihydroxy-5-[7-hydroxy-2-(4...)Show SMILES CCCCCCCCCCCC(=O)c1c(O)c(C2CC(Oc3cc(O)ccc23)c2ccc(O)cc2)c(O)c(C(=O)CCCCCCCCCCC)c1O Show InChI InChI=1S/C45H62O8/c1-3-5-7-9-11-13-15-17-19-21-36(48)41-43(50)40(44(51)42(45(41)52)37(49)22-20-18-16-14-12-10-8-6-4-2)35-30-38(31-23-25-32(46)26-24-31)53-39-29-33(47)27-28-34(35)39/h23-29,35,38,46-47,50-52H,3-22,30H2,1-2H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse sPLA2 group 5 |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50274337
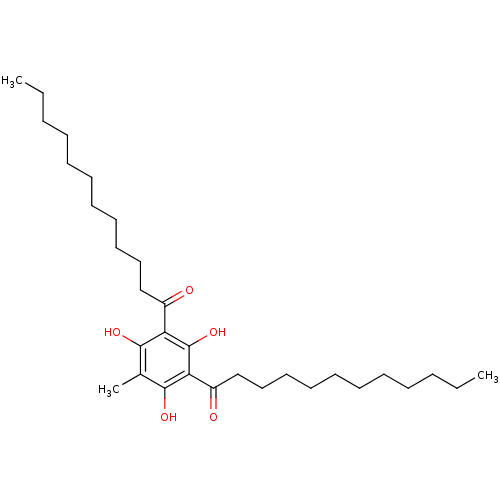
(1-(3-Dodecanoyl-2,4,6-trihydroxy-5-methyl-phenyl)-...)Show SMILES CCCCCCCCCCCC(=O)c1c(O)c(C)c(O)c(C(=O)CCCCCCCCCCC)c1O Show InChI InChI=1S/C31H52O5/c1-4-6-8-10-12-14-16-18-20-22-25(32)27-29(34)24(3)30(35)28(31(27)36)26(33)23-21-19-17-15-13-11-9-7-5-2/h34-36H,4-23H2,1-3H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2 group 5 |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50206911

((+/+)-1-O-decyl-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H...)Show SMILES CCCCCCCCCCCCCCOC(COCCCCCCCCCC)COc1ccc(cc1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C35H60N2O5/c1-3-5-7-9-11-13-14-15-16-18-20-22-28-40-33(29-39-27-21-19-17-12-10-8-6-4-2)30-41-32-25-23-31(24-26-32)34-36-35(38)42-37-34/h23-26,33H,3-22,27-30H2,1-2H3,(H,36,37,38) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM18207
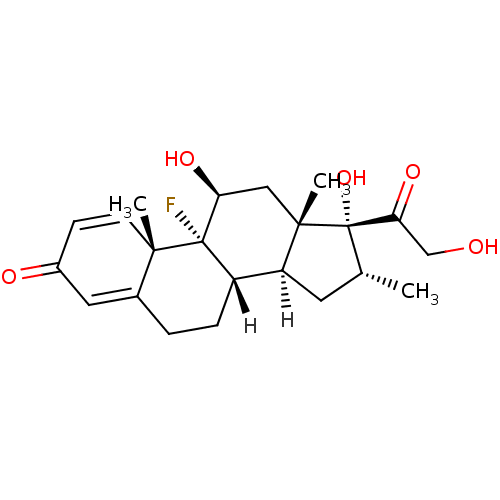
((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...)Show SMILES [H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24| Show InChI InChI=1S/C22H29FO5/c1-12-8-16-15-5-4-13-9-14(25)6-7-19(13,2)21(15,23)17(26)10-20(16,3)22(12,28)18(27)11-24/h6-7,9,12,15-17,24,26,28H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,17+,19+,20+,21+,22+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz
| Assay Description
Briefly, sn-2ester bond of the substrate 1,2-bis(heptanoylthio)-glycerophosphocholine was hydrolyzed by PLA2-V followed by the exposure of free thiol... |
Chem Biol Drug Des 85: 729-42 (2015)
Article DOI: 10.1111/cbdd.12457
BindingDB Entry DOI: 10.7270/Q26W98TX |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50206920

((+/-)-3-O-[4-(4,5-dihydro-5-oxo-1,2,4-4H-oxadiazol...)Show SMILES CCCCCCCCCCCCCCOC(COc1ccc(cc1)-c1nc(=O)o[nH]1)COC(c1ccccc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C44H54N2O5/c1-2-3-4-5-6-7-8-9-10-11-12-22-33-48-41(34-49-40-31-29-36(30-32-40)42-45-43(47)51-46-42)35-50-44(37-23-16-13-17-24-37,38-25-18-14-19-26-38)39-27-20-15-21-28-39/h13-21,23-32,41H,2-12,22,33-35H2,1H3,(H,45,46,47) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50458609
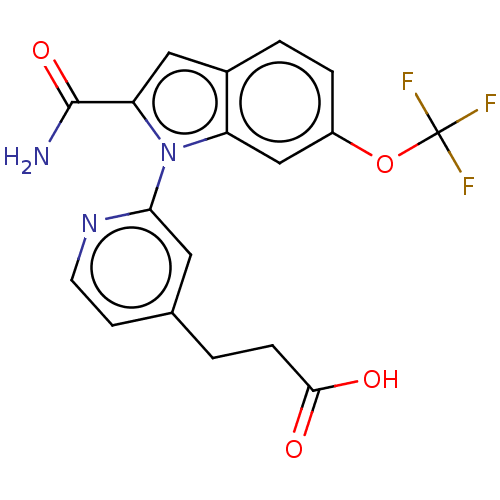
(CHEMBL4213094)Show SMILES NC(=O)c1cc2ccc(OC(F)(F)F)cc2n1-c1cc(CCC(O)=O)ccn1 Show InChI InChI=1S/C18H14F3N3O4/c19-18(20,21)28-12-3-2-11-8-14(17(22)27)24(13(11)9-12)15-7-10(5-6-23-15)1-4-16(25)26/h2-3,5-9H,1,4H2,(H2,22,27)(H,25,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... |
ACS Med Chem Lett 9: 600-605 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00507
BindingDB Entry DOI: 10.7270/Q2Z32272 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50186585

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2-ethyl-6-meth...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C22H22N2O5/c1-3-15-20(21(27)22(23)28)19-16(24(15)11-14-7-5-4-6-8-14)9-13(2)10-17(19)29-12-18(25)26/h4-10H,3,11-12H2,1-2H3,(H2,23,28)(H,25,26) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G5 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G5 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50055366

((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...)Show SMILES CCc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-2-14-19(20(26)21(22)27)18-15(9-6-10-16(18)28-12-17(24)25)23(14)11-13-7-4-3-5-8-13/h3-10H,2,11-12H2,1H3,(H2,22,27)(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50055383

((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C20H18N2O5/c1-12-17(19(25)20(21)26)18-14(8-5-9-15(18)27-11-16(23)24)22(12)10-13-6-3-2-4-7-13/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G5 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50186586
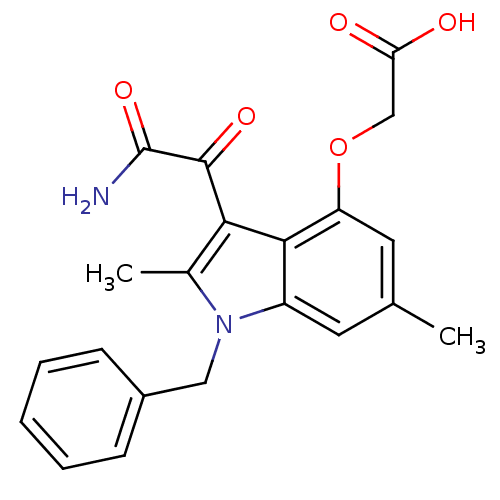
(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2,6-dimethyl-1...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-12-8-15-19(16(9-12)28-11-17(24)25)18(20(26)21(22)27)13(2)23(15)10-14-6-4-3-5-7-14/h3-9H,10-11H2,1-2H3,(H2,22,27)(H,24,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant sPLA2 G5 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50186586

(2-(3-(2-amino-2-oxoacetyl)-1-benzyl-2,6-dimethyl-1...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cc(C)cc2n1Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5/c1-12-8-15-19(16(9-12)28-11-17(24)25)18(20(26)21(22)27)13(2)23(15)10-14-6-4-3-5-7-14/h3-9H,10-11H2,1-2H3,(H2,22,27)(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G5 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
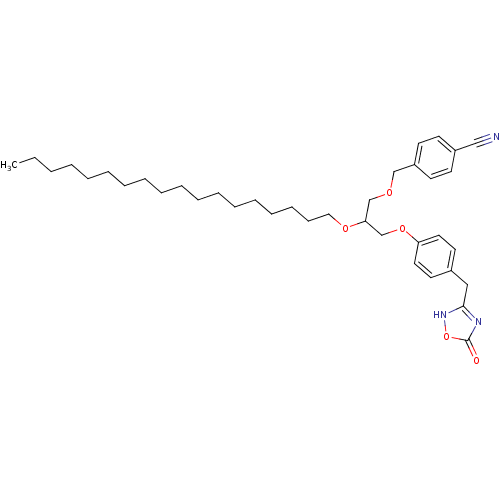
(Homo sapiens (Human)) | BDBM50206910
((+/-)-1-O-(4-cyanomethylphenyl)-3-O-[4-(4,5-dihydr...)Show SMILES CCCCCCCCCCCCCCCCCCOC(COCc1ccc(cc1)C#N)COc1ccc(Cc2nc(=O)o[nH]2)cc1 Show InChI InChI=1S/C38H55N3O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26-44-36(30-43-29-34-20-18-33(28-39)19-21-34)31-45-35-24-22-32(23-25-35)27-37-40-38(42)46-41-37/h18-25,36H,2-17,26-27,29-31H2,1H3,(H,40,41,42) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paris7-Denis Diderot
Curated by ChEMBL
| Assay Description
Inhibition of human group V PLA2 in [3H]oleate-labeled Escherichia coli membrane by radiometric assay |
J Med Chem 50: 1618-26 (2007)
Article DOI: 10.1021/jm060082n
BindingDB Entry DOI: 10.7270/Q2TQ6174 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50055383

((3-Aminooxalyl-1-benzyl-2-methyl-1H-indol-4-yloxy)...)Show SMILES Cc1c(C(=O)C(N)=O)c2c(OCC(O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C20H18N2O5/c1-12-17(19(25)20(21)26)18-14(8-5-9-15(18)27-11-16(23)24)22(12)10-13-6-3-2-4-7-13/h2-9H,10-11H2,1H3,(H2,21,26)(H,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant sPLA2 G5 |
J Med Chem 49: 2858-60 (2006)
Article DOI: 10.1021/jm060136t
BindingDB Entry DOI: 10.7270/Q26W99PJ |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50591714
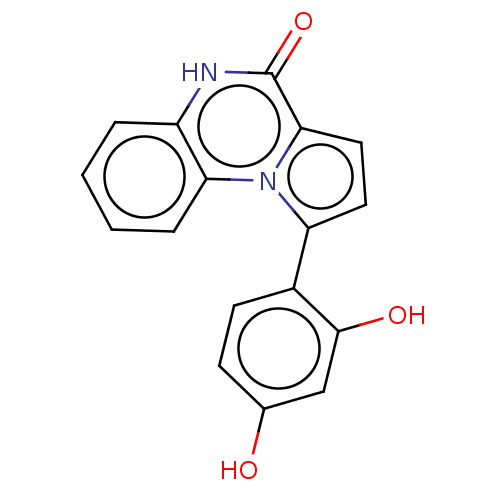
(CHEMBL5200953) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114085
BindingDB Entry DOI: 10.7270/Q2XW4PS2 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50149981
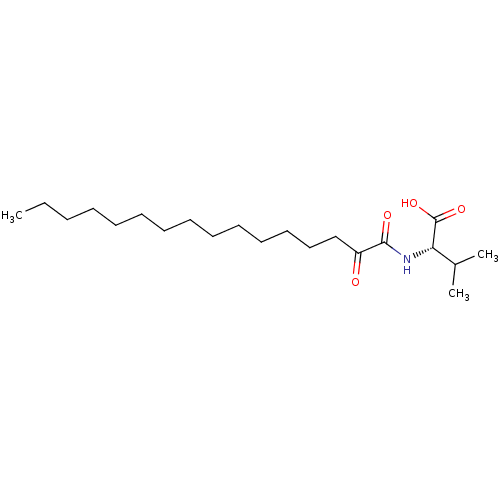
(CHEMBL3769794)Show SMILES CCCCCCCCCCCCCCC(=O)C(=O)N[C@@H](C(C)C)C(O)=O |r| Show InChI InChI=1S/C23H22BrN5O5S/c1-26-23(32)18(12-21(30)29-20-11-10-16(24)13-27-20)28-22(31)15-8-6-14(7-9-15)17-4-2-3-5-19(17)35(25,33)34/h2-11,13,18H,12H2,1H3,(H,26,32)(H,28,31)(H2,25,33,34)(H,27,29,30) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National and Kapodistrian University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of human group 5 secreted phospholipase A2 by fluorescence assay |
Bioorg Med Chem 24: 1683-95 (2016)
Article DOI: 10.1016/j.bmc.2016.02.040
BindingDB Entry DOI: 10.7270/Q2JQ12WP |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50591712
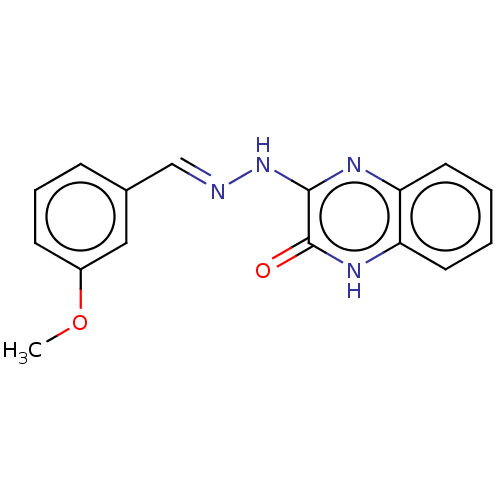
(CHEMBL5194852) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114085
BindingDB Entry DOI: 10.7270/Q2XW4PS2 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50333789

((R)-4-Methyl-2-(2-oxohexadecanamido)pentanoic acid...)Show SMILES CCCCCCCCCCCCCCC(=O)C(=O)N[C@H](CC(C)C)C(O)=O |r| Show InChI InChI=1S/C22H41NO4/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-20(24)21(25)23-19(22(26)27)17-18(2)3/h18-19H,4-17H2,1-3H3,(H,23,25)(H,26,27)/t19-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of human group 5 sPLA2 by fluorescence assay |
Bioorg Med Chem 19: 735-43 (2011)
Article DOI: 10.1016/j.bmc.2010.12.030
BindingDB Entry DOI: 10.7270/Q2JM29VG |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50591713

(CHEMBL5207115)Show SMILES [O-][N+](=O)c1ccc(-c2ccc3n2c2ccccc2[nH]c3=O)c(c1)[N+]([O-])=O | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114085
BindingDB Entry DOI: 10.7270/Q2XW4PS2 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50263053
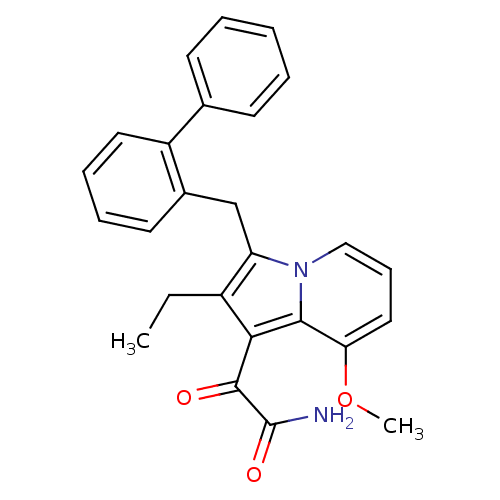
(2-(3-Biphenyl-2-ylmethyl-2-ethyl-8-methoxy-indoliz...)Show SMILES CCc1c(Cc2ccccc2-c2ccccc2)n2cccc(OC)c2c1C(=O)C(N)=O Show InChI InChI=1S/C26H24N2O3/c1-3-19-21(16-18-12-7-8-13-20(18)17-10-5-4-6-11-17)28-15-9-14-22(31-2)24(28)23(19)25(29)26(27)30/h4-15H,3,16H2,1-2H3,(H2,27,30) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human group2V phospholipase A2 fluorimetric assay |
J Med Chem 51: 4708-14 (2008)
Article DOI: 10.1021/jm800422v
BindingDB Entry DOI: 10.7270/Q2571BSD |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50274388
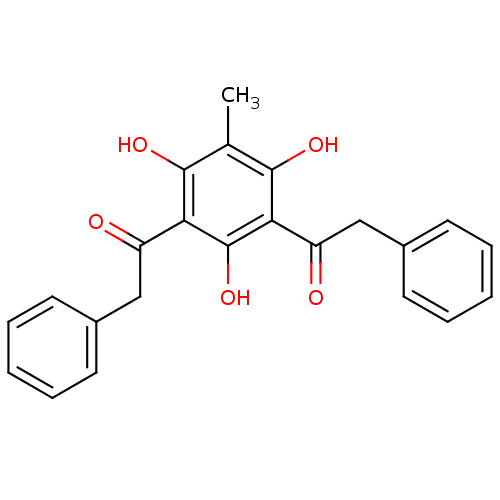
(2-Phenyl-1-(2,4,6-trihydroxy-3-methyl-5-phenylacet...)Show SMILES Cc1c(O)c(C(=O)Cc2ccccc2)c(O)c(C(=O)Cc2ccccc2)c1O Show InChI InChI=1S/C23H20O5/c1-14-21(26)19(17(24)12-15-8-4-2-5-9-15)23(28)20(22(14)27)18(25)13-16-10-6-3-7-11-16/h2-11,26-28H,12-13H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse sPLA2 group 5 |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50274389
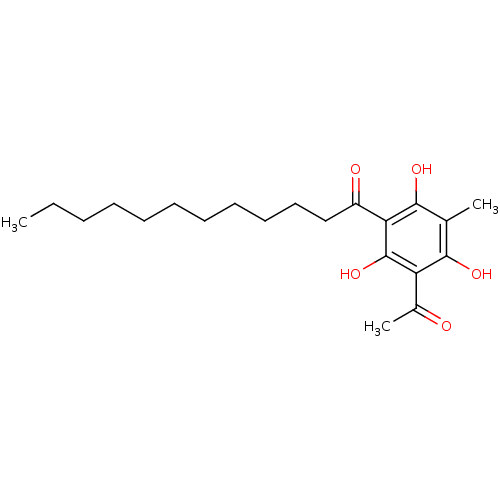
(1-(3-acetyl-2,4,6-trihydroxy-5-methylphenyl)dodeca...)Show InChI InChI=1S/C21H32O5/c1-4-5-6-7-8-9-10-11-12-13-16(23)18-20(25)14(2)19(24)17(15(3)22)21(18)26/h24-26H,4-13H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse sPLA2 group 5 |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50274387

(1-(3-Acetyl-2,4,6-trihydroxy-5-methyl-phenyl)-etha...)Show InChI InChI=1S/C11H12O5/c1-4-9(14)7(5(2)12)11(16)8(6(3)13)10(4)15/h14-16H,1-3H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2 group 5 |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50274388

(2-Phenyl-1-(2,4,6-trihydroxy-3-methyl-5-phenylacet...)Show SMILES Cc1c(O)c(C(=O)Cc2ccccc2)c(O)c(C(=O)Cc2ccccc2)c1O Show InChI InChI=1S/C23H20O5/c1-14-21(26)19(17(24)12-15-8-4-2-5-9-15)23(28)20(22(14)27)18(25)13-16-10-6-3-7-11-16/h2-11,26-28H,12-13H2,1H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2 group 5 |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50274389

(1-(3-acetyl-2,4,6-trihydroxy-5-methylphenyl)dodeca...)Show InChI InChI=1S/C21H32O5/c1-4-5-6-7-8-9-10-11-12-13-16(23)18-20(25)14(2)19(24)17(15(3)22)21(18)26/h24-26H,4-13H2,1-3H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2 group 5 |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Mus musculus) | BDBM50274441
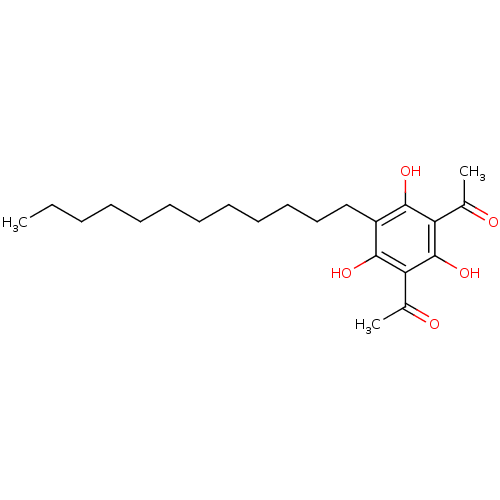
(1-(3-Acetyl-5-dodecyl-2,4,6-trihydroxy-phenyl)-eth...)Show SMILES CCCCCCCCCCCCc1c(O)c(C(C)=O)c(O)c(C(C)=O)c1O Show InChI InChI=1S/C22H34O5/c1-4-5-6-7-8-9-10-11-12-13-14-17-20(25)18(15(2)23)22(27)19(16(3)24)21(17)26/h25-27H,4-14H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of mouse sPLA2 group 5 |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |
Phospholipase A2 group V
(Homo sapiens (Human)) | BDBM50274441

(1-(3-Acetyl-5-dodecyl-2,4,6-trihydroxy-phenyl)-eth...)Show SMILES CCCCCCCCCCCCc1c(O)c(C(C)=O)c(O)c(C(C)=O)c1O Show InChI InChI=1S/C22H34O5/c1-4-5-6-7-8-9-10-11-12-13-14-17-20(25)18(15(2)23)22(27)19(16(3)24)21(17)26/h25-27H,4-14H2,1-3H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington
Curated by ChEMBL
| Assay Description
Inhibition of human sPLA2 group 5 |
Bioorg Med Chem Lett 18: 5415-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.041
BindingDB Entry DOI: 10.7270/Q2CJ8DB1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data