

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-type tyrosine-protein phosphatase eta (Homo sapiens (Human)) | BDBM231167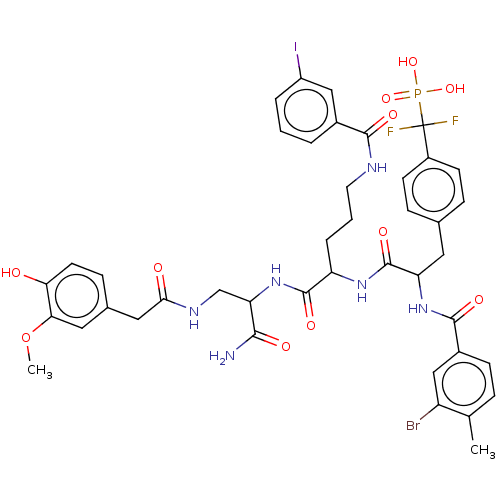 (US9340574, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 ... | US Patent US9340574 (2016) BindingDB Entry DOI: 10.7270/Q2NV9H4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase eta (Homo sapiens (Human)) | BDBM50544440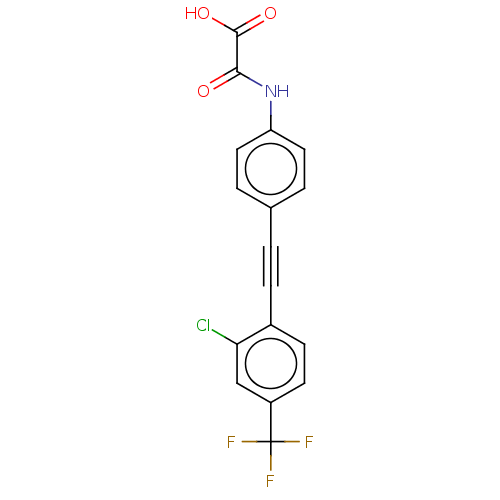 (CHEMBL4647367 | US11192850, Entry 4t) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of DEP1 (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase eta (Homo sapiens (Human)) | BDBM50544431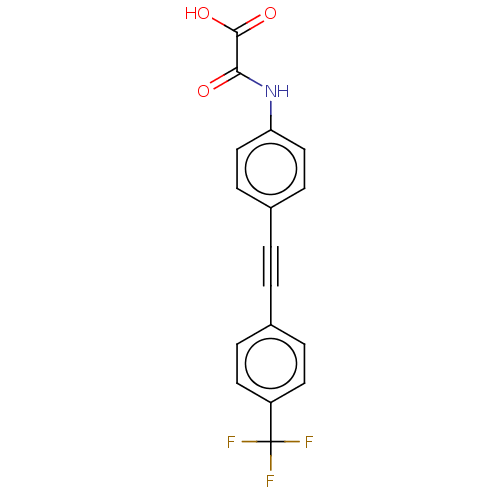 (CHEMBL4637459 | US11192850, Entry 4k) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of DEP1 (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase eta (Homo sapiens (Human)) | BDBM50544427 (CHEMBL4632818 | US11192850, Entry 4g) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of DEP1 (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vis sp... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase eta (Homo sapiens (Human)) | BDBM152210 (US8987474, NSC-87877) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida; H. Lee Moffitt Cancer Center and Research Institute, Inc. US Patent | Assay Description NSC-87877 ranked among top 10% (175th) of the compounds with the best GLIDE scores for the docking to the human Shp2 PTP domain in our virtual screen... | US Patent US8987474 (2015) BindingDB Entry DOI: 10.7270/Q2ZS2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase eta (Homo sapiens (Human)) | BDBM50071058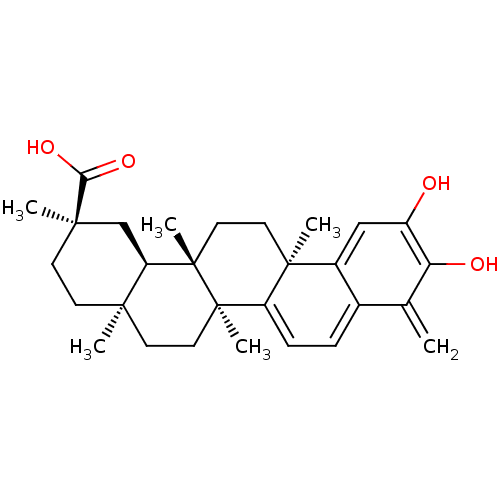 ((2R,4aS,6aS,12bR,14aS,14bR)-10-Hydroxy-2,4a,6a,9,1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz Zentrum M�nchen Curated by ChEMBL | Assay Description Inhibition of human PTPRJ (1019 to 1311 residues) expressed in Escherichia coli strain BL21(DE3) using DiFMUP as substrate preincubated for 10 mins f... | J Med Chem 61: 11144-11157 (2018) Article DOI: 10.1021/acs.jmedchem.8b01224 BindingDB Entry DOI: 10.7270/Q2G44TN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||