

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50367247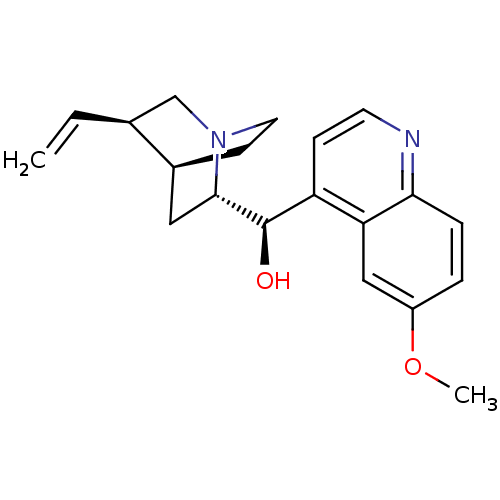 (QUININE | Quinamm | Quinsan | cid_3034034) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM112777 (NORTRIPTYLINE | US8629135, SW-02) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50444436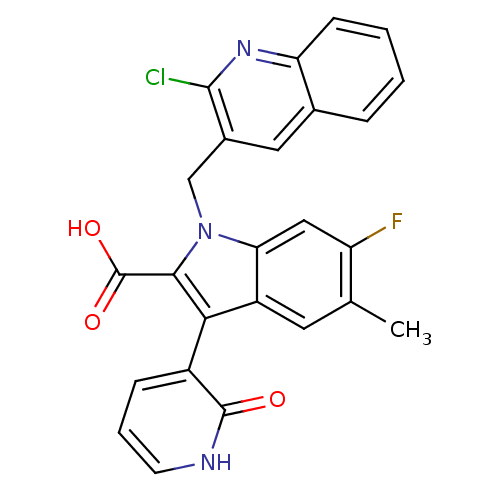 (CHEMBL3092124) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat CYP2D4 measured after concurrent incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50444436 (CHEMBL3092124) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat CYP2D4 measured after compound pre-incubation | Bioorg Med Chem Lett 23: 6585-7 (2013) Article DOI: 10.1016/j.bmcl.2013.10.060 BindingDB Entry DOI: 10.7270/Q2GF0W06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50426305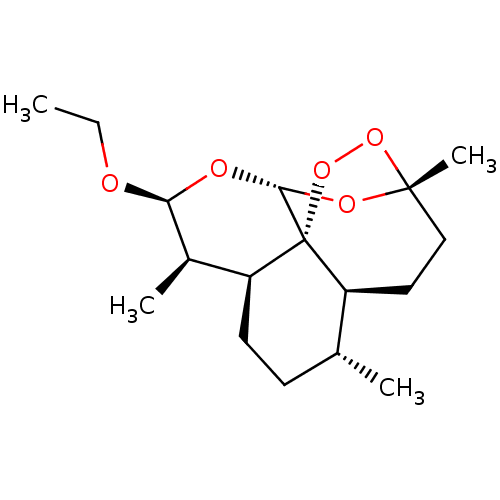 (ARTEMOTIL) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Medicinal& Process Chemistry, Division of Parasitology, Division of Pharmacokinetics and Metabolism, and Sophisticated Analytical Instrument Facility, Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of CYP2D4 in rat liver microsome using dextromethorphan as substrate by HPLC-PDA analysis | ACS Med Chem Lett 4: 165-9 (2013) Article DOI: 10.1021/ml300188t BindingDB Entry DOI: 10.7270/Q2V1264R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM36108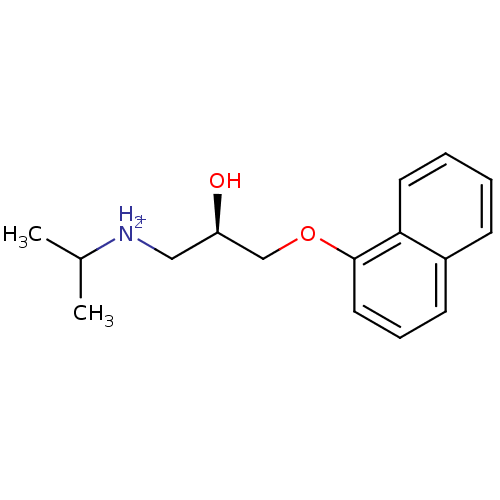 ((R)-propranolol) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50246936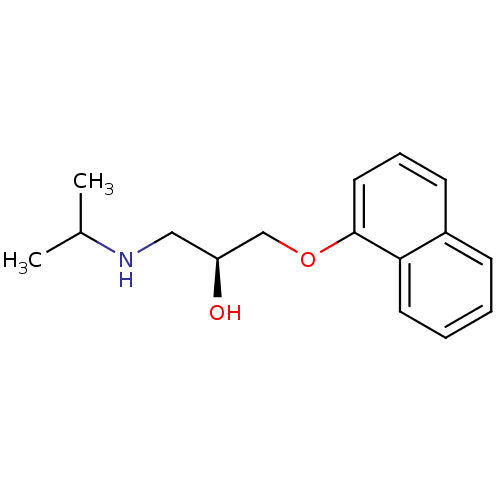 ((-)-(S)-Propranolol | 1-(ISOPROPYLAMINO)-3-(1-NAPH...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50366613 (DEXTROMETHORPHAN) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50122613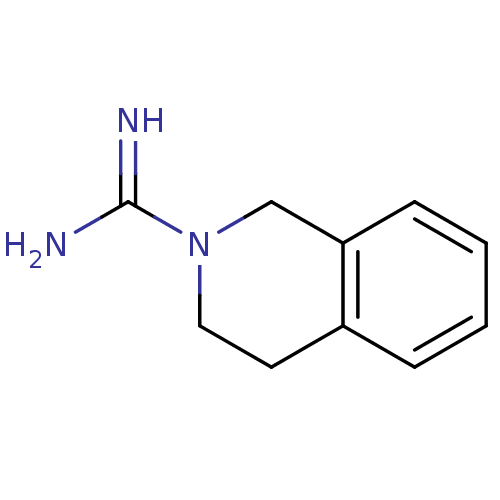 (3,4-dihydroisoquinoline-2(1H)-carboximidamide | CH...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50240908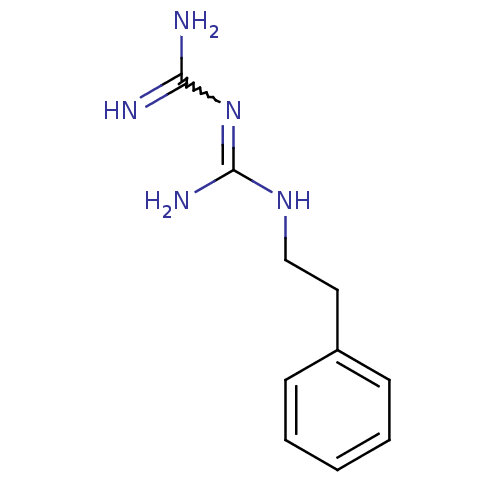 (CHEMBL170988 | N''-[(E)-amino(imino)methyl]-N-(2-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50122614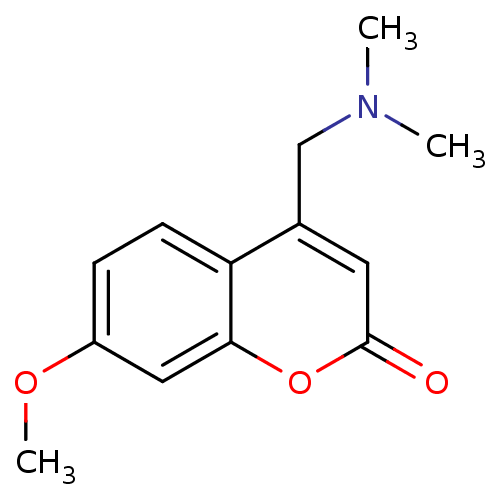 (4-Dimethylaminomethyl-7-methoxy-chromen-2-one | CH...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50122615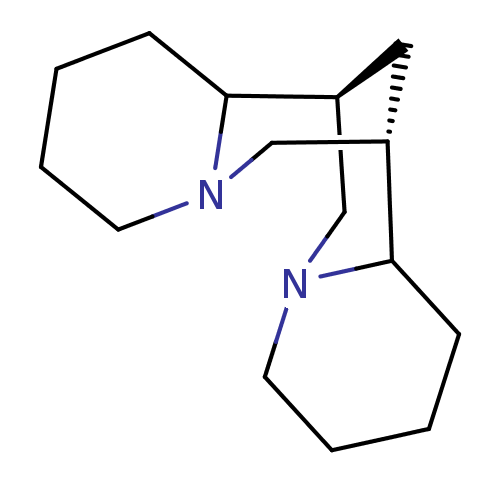 (CHEMBL2373353 | Dodecahydro-7,14-methano-dipyrido[...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D4 (Rattus norvegicus) | BDBM50019351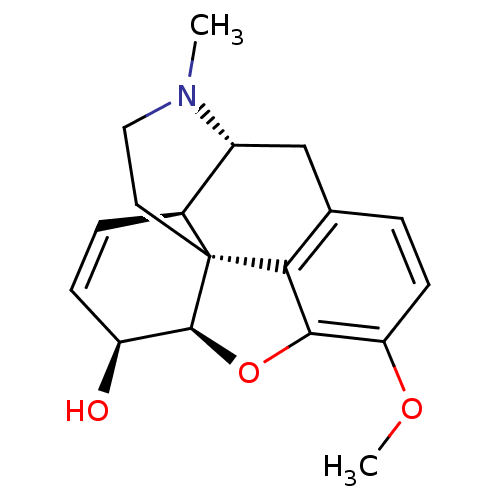 ((-)-Codeine | (Codeine) | (codeine)10-methoxy-4-me...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.22E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Curated by ChEMBL | Assay Description Inhibition of MAMC O-dealkylation mediated by rat Cytochrome P450 2D4 expressed in Saccharomyces cerevisiae | J Med Chem 46: 74-86 (2002) Article DOI: 10.1021/jm0209578 BindingDB Entry DOI: 10.7270/Q2K64JSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||