

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM600744 (US11629156, Example 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM603024 (1-Ethyl-7-[[(1S)-1-[4- [(1R/S)-1-(4-prop-2- enoylp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497343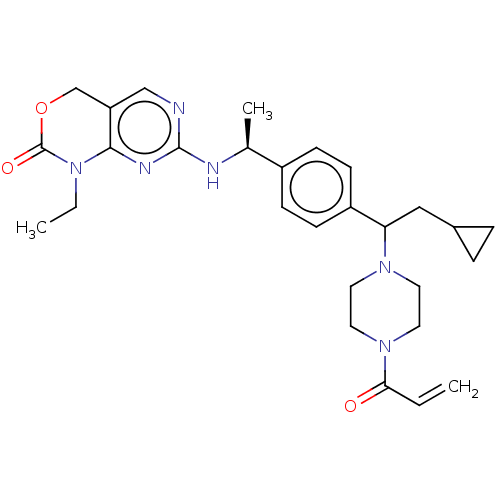 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.49 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497343 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas... | US Patent US11001596 (2021) BindingDB Entry DOI: 10.7270/Q21V5J2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497343 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.49 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497335 (7-[[(1S)-1-[4-[(1S)-2- cyclopropyl-1-(4-prop-2- en...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 3.71 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas... | US Patent US11001596 (2021) BindingDB Entry DOI: 10.7270/Q21V5J2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497335 (7-[[(1S)-1-[4-[(1S)-2- cyclopropyl-1-(4-prop-2- en...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 3.71 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497335 (7-[[(1S)-1-[4-[(1S)-2- cyclopropyl-1-(4-prop-2- en...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | n/a | n/a | 3.71 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine | Assay Description RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates... | Nat Chem Biol 11: 878-86 (2015) Article DOI: 10.1038/nchembio.1930 BindingDB Entry DOI: 10.7270/Q2RR1X2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound 1 was not initially reported as having the greatest biochemical potency compared to reports for certain other small molecule inhibitors of m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DF6W4N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497334 (7-[[(1S)-1-[4-[(1R)-2-Cyclopropyl-1-(4-prop-2-enoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 4.31 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497334 (7-[[(1S)-1-[4-[(1R)-2-Cyclopropyl-1-(4-prop-2-enoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas... | US Patent US11001596 (2021) BindingDB Entry DOI: 10.7270/Q21V5J2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497334 (7-[[(1S)-1-[4-[(1R)-2-Cyclopropyl-1-(4-prop-2-enoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 4.31 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497348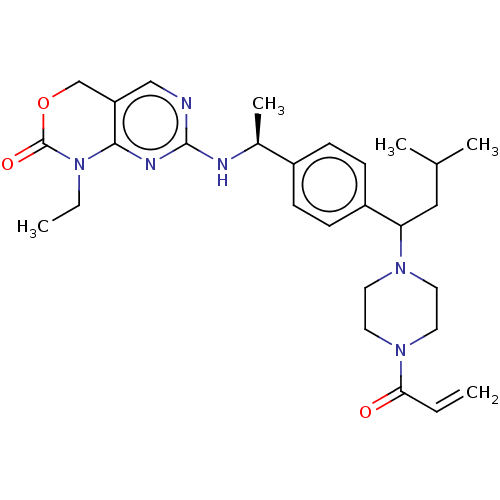 (1-Ethyl-7-[[(1S)-1-[4-[3- methyl-1-(4-prop-2- enoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas... | US Patent US11001596 (2021) BindingDB Entry DOI: 10.7270/Q21V5J2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497348 (1-Ethyl-7-[[(1S)-1-[4-[3- methyl-1-(4-prop-2- enoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497348 (1-Ethyl-7-[[(1S)-1-[4-[3- methyl-1-(4-prop-2- enoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 5.05 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM448752 ((4S)-3-[2-[[(1S)-1-[4-[2-Cyclopropyl-1-(4-prop-2-e...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1 mutant (R132H and R132C) and IDH2 mutant (R140Q and R172K) proteins containing N-terminal His-tag are expressed in E. coli and purified using ni... | US Patent US10696665 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM448754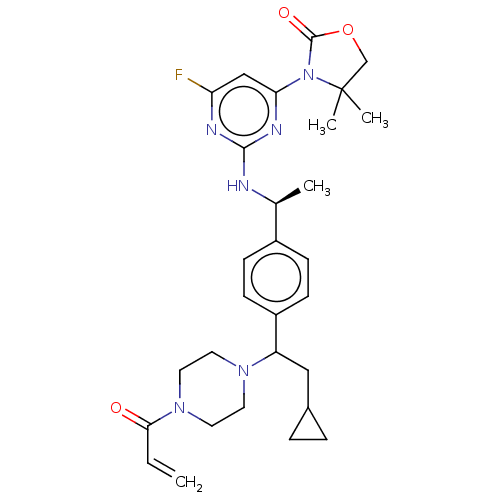 (3-[2-[[(1S)-1-[4-[2-Cyclopropyl-1-(4-prop-2-enoylp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1 mutant (R132H and R132C) and IDH2 mutant (R140Q and R172K) proteins containing N-terminal His-tag are expressed in E. coli and purified using ni... | US Patent US10696665 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497348 (1-Ethyl-7-[[(1S)-1-[4-[3- methyl-1-(4-prop-2- enoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas... | US Patent US11001596 (2021) BindingDB Entry DOI: 10.7270/Q21V5J2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497348 (1-Ethyl-7-[[(1S)-1-[4-[3- methyl-1-(4-prop-2- enoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas... | US Patent US11001596 (2021) BindingDB Entry DOI: 10.7270/Q21V5J2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM448752 ((4S)-3-[2-[[(1S)-1-[4-[2-Cyclopropyl-1-(4-prop-2-e...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1 mutant (R132H and R132C) and IDH2 mutant (R140Q and R172K) proteins containing N-terminal His-tag are expressed in E. coli and purified using ni... | US Patent US10696665 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497348 (1-Ethyl-7-[[(1S)-1-[4-[3- methyl-1-(4-prop-2- enoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <5.08 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM600741 (US11629156, Example 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <5.08 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM600740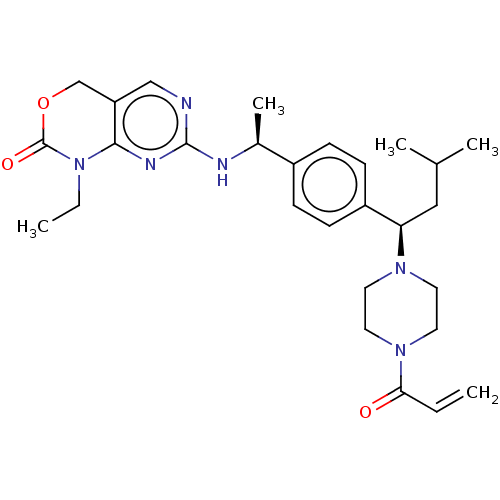 (US11629156, Example 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <5.08 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497348 (1-Ethyl-7-[[(1S)-1-[4-[3- methyl-1-(4-prop-2- enoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <5.08 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM50506474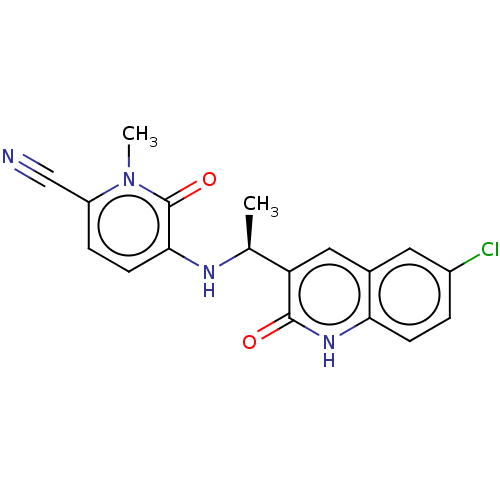 (Ft-2102 | Olutasidenib | US11311527, Cpd ID 1 | US...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound 1 was not initially reported as having the greatest biochemical potency compared to reports for certain other small molecule inhibitors of m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DF6W4N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM600742 (US11629156, Example 4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8.69 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497337 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.69 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas... | US Patent US11001596 (2021) BindingDB Entry DOI: 10.7270/Q21V5J2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM603011 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 8.69 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM50503281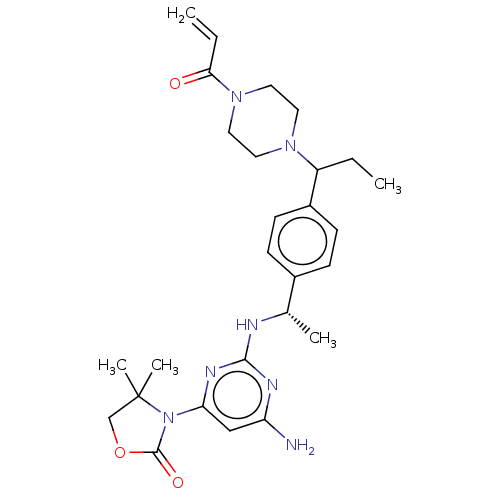 (CHEMBL4586919 | US10696665, Example 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.89 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1 mutant (R132H and R132C) and IDH2 mutant (R140Q and R172K) proteins containing N-terminal His-tag are expressed in E. coli and purified using ni... | US Patent US10696665 (2020) BindingDB Entry DOI: 10.7270/Q2ZK5KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM375167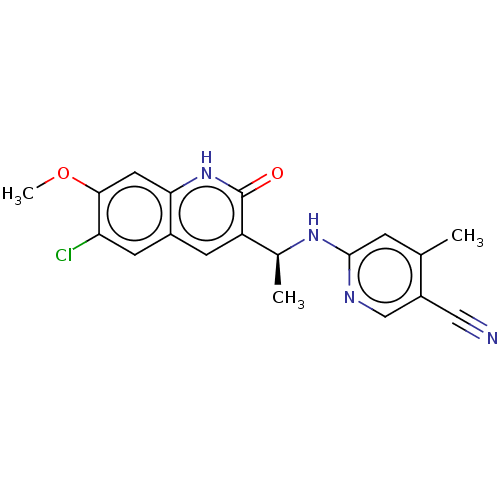 (6-{[(1S)-1-(6-chloro-7- | US10253015, Compound I-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine | Assay Description Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13... | Proc Natl Acad Sci U S A 97: 925-30 (2000) BindingDB Entry DOI: 10.7270/Q23F4S02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM375163 (6-{[(1S)-1-(6-chloro-7- | US10253015, Compound I-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine | Assay Description Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13... | Proc Natl Acad Sci U S A 97: 925-30 (2000) BindingDB Entry DOI: 10.7270/Q23F4S02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM375168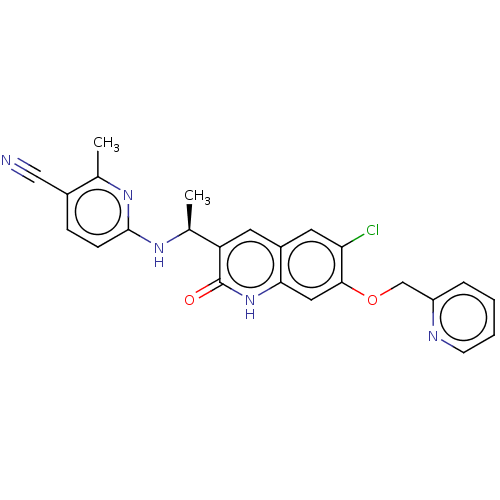 (6-{[(1S)-1-[6-chloro-2-oxo- | US10253015, Compound...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine | Assay Description HCT116 isogenic IDH1-R132H and IDH1-R132C mutant cells were cultured in growth media (McCoy's 5 A, 10% fetal bovine serum, 1× antibiotic-antimyco... | Proc Natl Acad Sci U S A 97: 925-30 (2000) BindingDB Entry DOI: 10.7270/Q23F4S02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM375166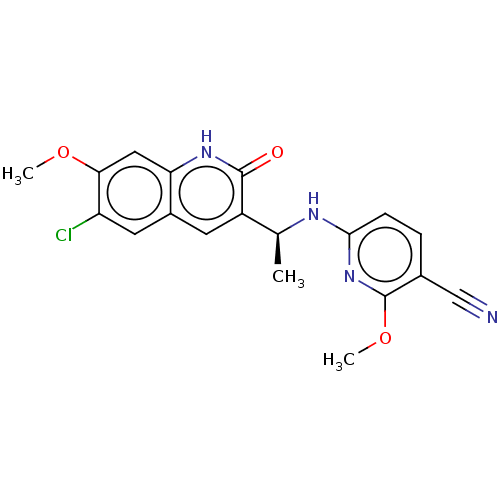 (6-{[(1S)-1-(6-chloro-7- | US10253015, Compound I-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine | Assay Description Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13... | Proc Natl Acad Sci U S A 97: 925-30 (2000) BindingDB Entry DOI: 10.7270/Q23F4S02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM375168 (6-{[(1S)-1-[6-chloro-2-oxo- | US10253015, Compound...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine | Assay Description Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13... | Proc Natl Acad Sci U S A 97: 925-30 (2000) BindingDB Entry DOI: 10.7270/Q23F4S02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM375165 (6-{[(1S)-1-(6-chloro-7- | US10253015, Compound I-2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine | Assay Description Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13... | Proc Natl Acad Sci U S A 97: 925-30 (2000) BindingDB Entry DOI: 10.7270/Q23F4S02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM592061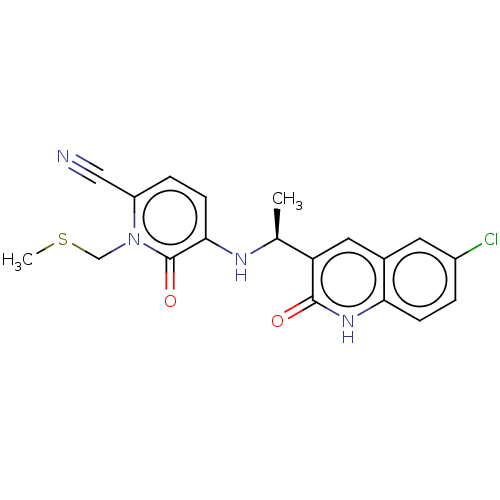 (US11566013, Compound I-1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Assays were performed in a 384-well black plate. An aliquot of 250 nL of compound was incubated with 10 μL of 30 nM IDH1-R132H or 10 nM IDH1-R13... | Citation and Details BindingDB Entry DOI: 10.7270/Q26W9G0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM363689 (US10640534, Compound 176 | US10682352, Compound AG...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound 1 was not initially reported as having the greatest biochemical potency compared to reports for certain other small molecule inhibitors of m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DF6W4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM404661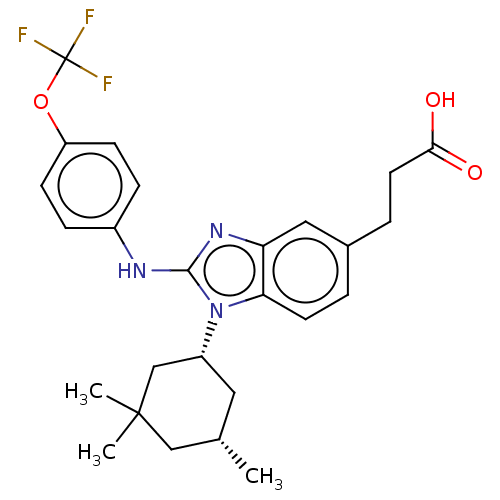 (US10344004, Test compound Table 3 | US10442772, Ex...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compound 1 was not initially reported as having the greatest biochemical potency compared to reports for certain other small molecule inhibitors of m... | Citation and Details BindingDB Entry DOI: 10.7270/Q2DF6W4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497351 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM603020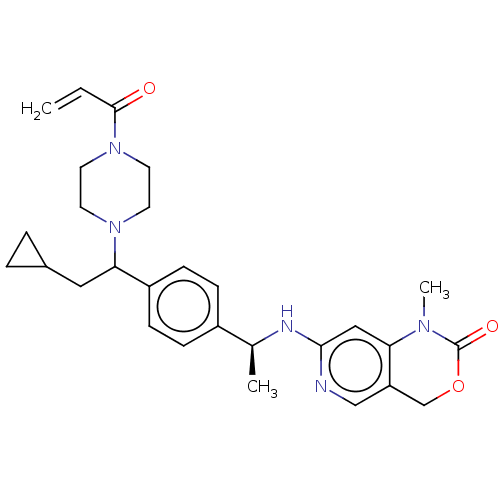 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497351 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas... | US Patent US11001596 (2021) BindingDB Entry DOI: 10.7270/Q21V5J2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497353 (1-Ethyl-7-[[(1S)-1-[4- [(1R/S)-1-(4-prop-2- enoylp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas... | US Patent US11001596 (2021) BindingDB Entry DOI: 10.7270/Q21V5J2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM603024 (1-Ethyl-7-[[(1S)-1-[4- [(1R/S)-1-(4-prop-2- enoylp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497337 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497337 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company US Patent | Assay Description IDH1-R132H, IDH1-R132C, IDH2-R172K and IDH2-R140Q mutant enzymes catalyze the conversion of αKG to 2HG. 2HG is analyzed using in-line solid phas... | US Patent US11001596 (2021) BindingDB Entry DOI: 10.7270/Q21V5J2Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM603011 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM497353 (1-Ethyl-7-[[(1S)-1-[4- [(1R/S)-1-(4-prop-2- enoylp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 15.6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM600743 (US11629156, Example 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2MK6HT9 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM603020 (7-[[(1S)-1-[4-[2- Cyclopropyl-1-(4-prop-2- enoylpi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 16.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DKW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2342 total ) | Next | Last >> |