

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50280455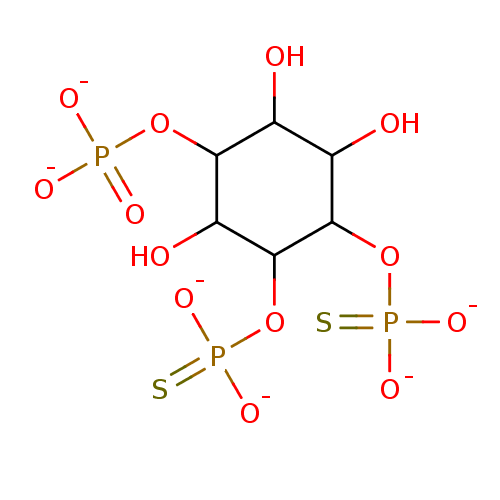 (Phosphoric acid mono-(2,3,6-trihydroxy-4,5-bis-thi...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of Inositol-1,4,5-trisphosphate 5-phosphatase | Bioorg Med Chem Lett 2: 471-476 (1992) Article DOI: 10.1016/S0960-894X(00)80172-9 BindingDB Entry DOI: 10.7270/Q2RR1ZQD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50407003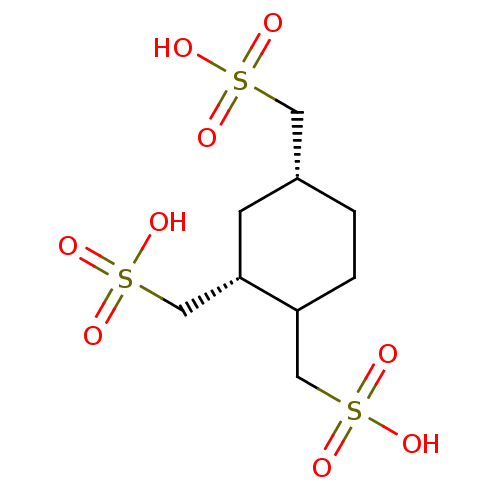 (CHEMBL2092837) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Tested for inhibition of 5-phosphatase isolated from human erythrocyte membrane | J Med Chem 36: 3035-8 (1993) BindingDB Entry DOI: 10.7270/Q2T72J2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50046068 (1D-3-deoxy- 1D-myo-Inositol 1,4,5-trisphosphate) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Tested for inhibition of 5-phosphatase isolated from human erythrocyte membrane (S substrate) | J Med Chem 36: 3035-8 (1993) BindingDB Entry DOI: 10.7270/Q2T72J2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50075183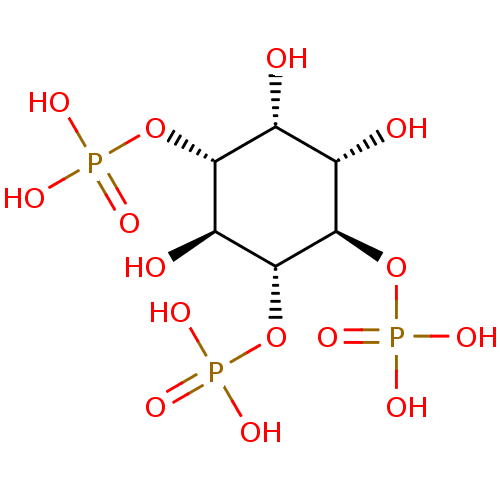 (1,4,5-Insp3 | 1D-myo-inositol 1,4,5-triphosphate |...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Tested for inhibition of 5-phosphatase isolated from human erythrocyte membrane (S substrate) | J Med Chem 36: 3035-8 (1993) BindingDB Entry DOI: 10.7270/Q2T72J2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50046075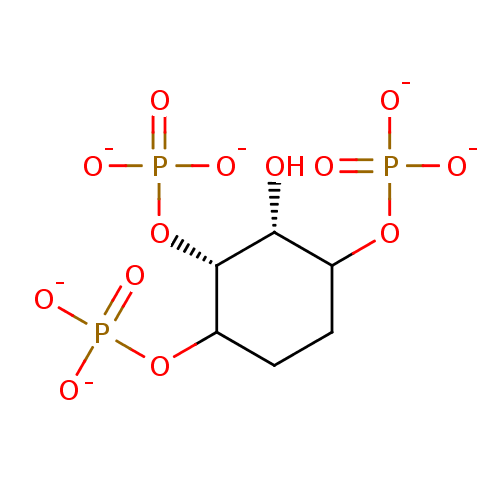 (1D-2,3,6--trioxy- 1D-myo-Inositol 1,4,5-trisphosph...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Tested for inhibition of 5-phosphatase isolated from human erythrocyte membrane (S substrate) | J Med Chem 36: 3035-8 (1993) BindingDB Entry DOI: 10.7270/Q2T72J2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50279840 ((D)-2,2-difluoro-2-deoxy-myo-inositol 1,4,5-tripho...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity towards Inositol-1,4,5-trisphosphate 5-phosphatase | Bioorg Med Chem Lett 1: 705-710 (1991) Article DOI: 10.1016/S0960-894X(01)81052-0 BindingDB Entry DOI: 10.7270/Q2348KV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50046074 (1D-2,3,6--trideoxy- 1D-myo-Inositol 1,4,5-trisphos...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 8.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Tested for inhibition of 5-phosphatase isolated from human erythrocyte membrane (S substrate) | J Med Chem 36: 3035-8 (1993) BindingDB Entry DOI: 10.7270/Q2T72J2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50046070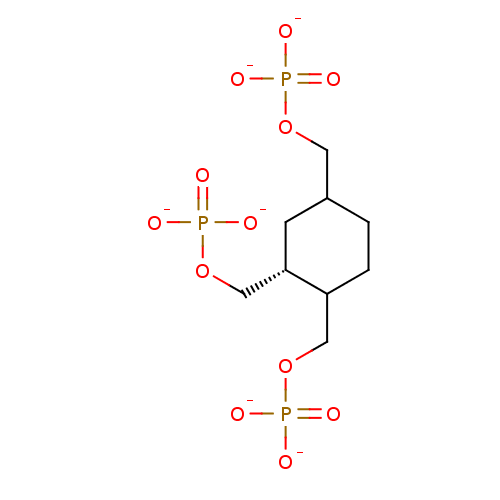 ((1R,2R,4R)-Cyclohexane-1,2,4-tris (methlyenephosph...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Tested for inhibition of 5-phosphatase isolated from human erythrocyte membrane (R metabolically resistant) | J Med Chem 36: 3035-8 (1993) BindingDB Entry DOI: 10.7270/Q2T72J2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50046073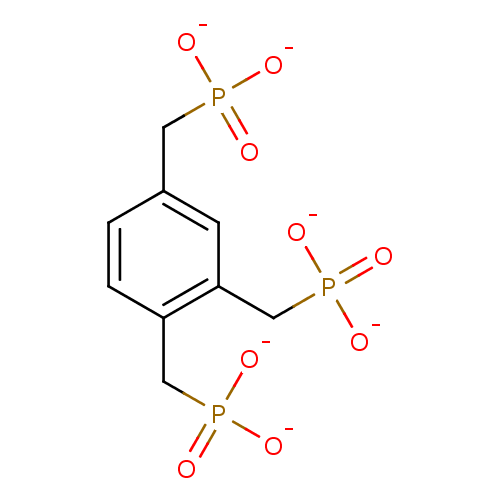 (Benzene-1,2,4-tris (methylenephosphonate)) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Tested for inhibition of 5-phosphatase isolated from human erythrocyte membrane (R metabolically resistant) | J Med Chem 36: 3035-8 (1993) BindingDB Entry DOI: 10.7270/Q2T72J2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50046072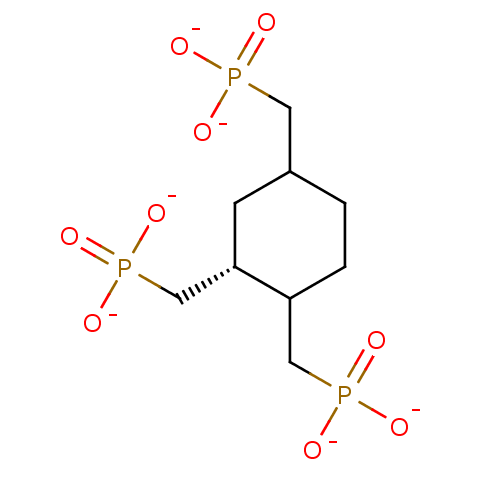 ((1R,2R,4R)-Cyclohexane-1,2,4-tris (methylenephosph...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 5.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Tested for inhibition of 5-phosphatase isolated from human erythrocyte membrane (R metabolically resistant) | J Med Chem 36: 3035-8 (1993) BindingDB Entry DOI: 10.7270/Q2T72J2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50407004 (CHEMBL2092922) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | >1.00E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Medical Education and Research Curated by ChEMBL | Assay Description Tested for inhibition of 5-phosphatase isolated from human erythrocyte membrane (R metabolically resistant) | J Med Chem 36: 3035-8 (1993) BindingDB Entry DOI: 10.7270/Q2T72J2R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50511342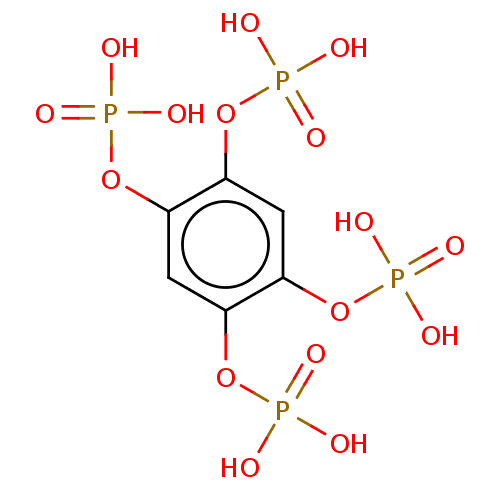 (CHEMBL4538474) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Biological Sciences, UEA Curated by ChEMBL | Assay Description Inhibition of recombinant human brain INPP5A expressed in Escherichia coli incubated for 10 mins by malachite green reagent based phosphate assay | ACS Med Chem Lett 11: 309-315 (2020) Article DOI: 10.1021/acsmedchemlett.9b00368 BindingDB Entry DOI: 10.7270/Q2BP063F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50511343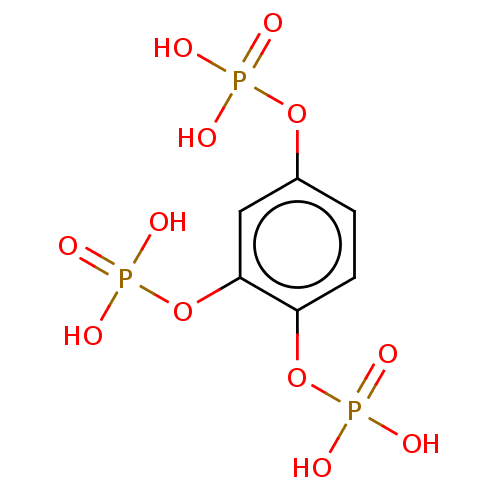 (CHEMBL4473747) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Biological Sciences, UEA Curated by ChEMBL | Assay Description Inhibition of recombinant human brain INPP5A expressed in Escherichia coli incubated for 10 mins by malachite green reagent based phosphate assay | ACS Med Chem Lett 11: 309-315 (2020) Article DOI: 10.1021/acsmedchemlett.9b00368 BindingDB Entry DOI: 10.7270/Q2BP063F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50511344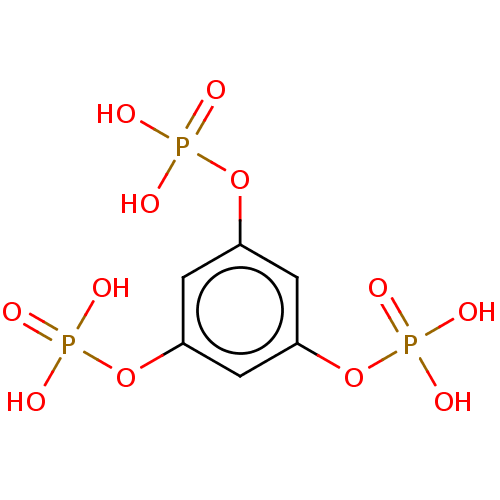 (CHEMBL4453163) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Biological Sciences, UEA Curated by ChEMBL | Assay Description Inhibition of recombinant human brain INPP5A expressed in Escherichia coli incubated for 10 mins by malachite green reagent based phosphate assay | ACS Med Chem Lett 11: 309-315 (2020) Article DOI: 10.1021/acsmedchemlett.9b00368 BindingDB Entry DOI: 10.7270/Q2BP063F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50511346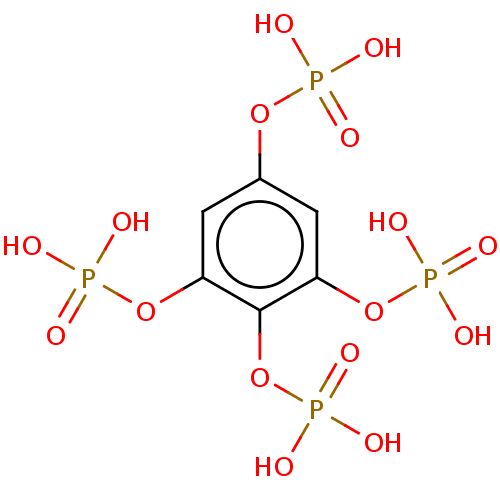 (CHEMBL4454154) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Biological Sciences, UEA Curated by ChEMBL | Assay Description Inhibition of recombinant human brain INPP5A expressed in Escherichia coli incubated for 10 mins by malachite green reagent based phosphate assay | ACS Med Chem Lett 11: 309-315 (2020) Article DOI: 10.1021/acsmedchemlett.9b00368 BindingDB Entry DOI: 10.7270/Q2BP063F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50511345 (CHEMBL4566548) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Biological Sciences, UEA Curated by ChEMBL | Assay Description Inhibition of recombinant human brain INPP5A expressed in Escherichia coli incubated for 10 mins by malachite green reagent based phosphate assay | ACS Med Chem Lett 11: 309-315 (2020) Article DOI: 10.1021/acsmedchemlett.9b00368 BindingDB Entry DOI: 10.7270/Q2BP063F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol polyphosphate-5-phosphatase A (Homo sapiens (Human)) | BDBM50304360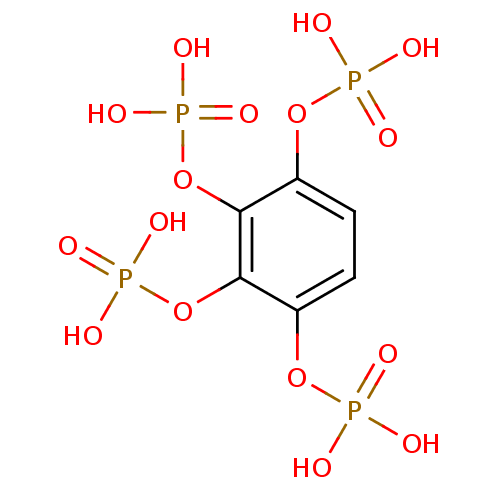 (BENZENE-1,2,3,4-TETRAYL TETRAKIS[DIHYDROGEN (PHOSP...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Biological Sciences, UEA Curated by ChEMBL | Assay Description Inhibition of recombinant human brain INPP5A expressed in Escherichia coli incubated for 10 mins by malachite green reagent based phosphate assay | ACS Med Chem Lett 11: 309-315 (2020) Article DOI: 10.1021/acsmedchemlett.9b00368 BindingDB Entry DOI: 10.7270/Q2BP063F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||