

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496713 (CHEMBL1715624) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496712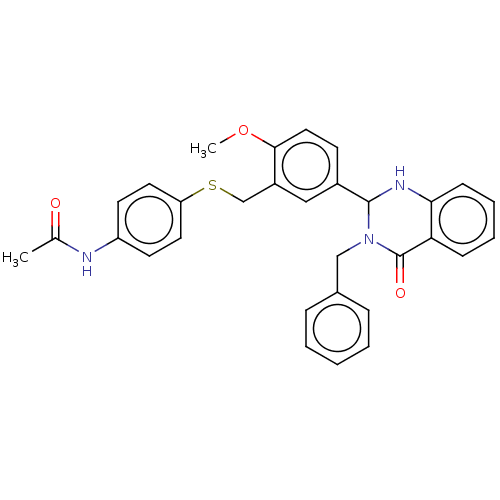 (CHEMBL1719708) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496706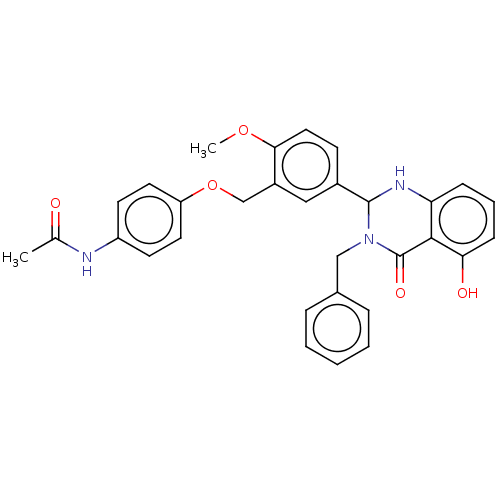 (CHEMBL1515891) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496707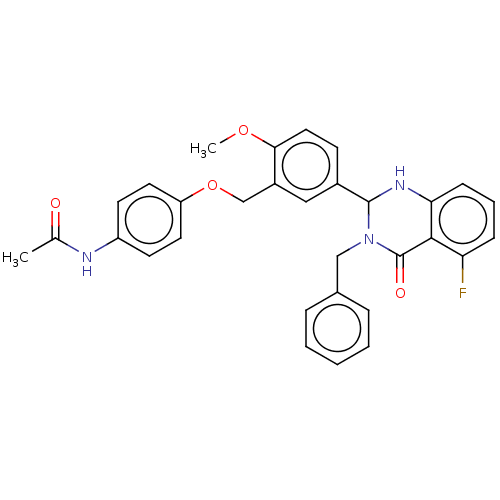 (CHEMBL1708839) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496702 (CHEMBL1331194) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 290 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496715 (CHEMBL1705547) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496703 (CHEMBL1589735) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496714 (CHEMBL1718442) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 550 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496708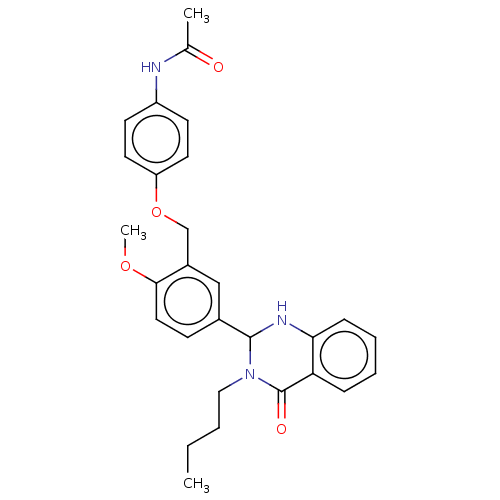 (CHEMBL1315387) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 830 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496705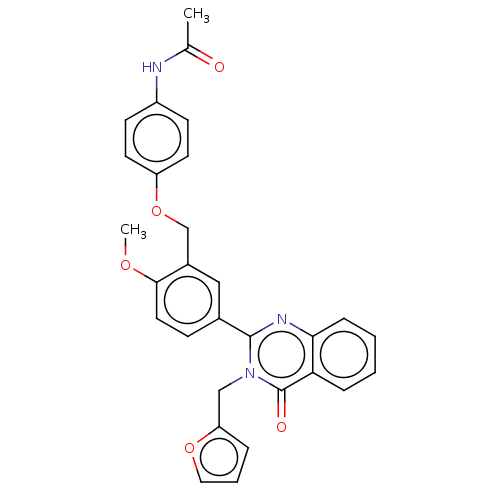 (CHEMBL1370580) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 930 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496717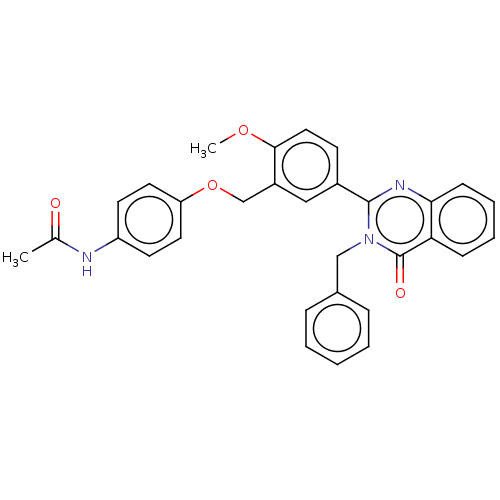 (CHEMBL1357440) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 960 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM83388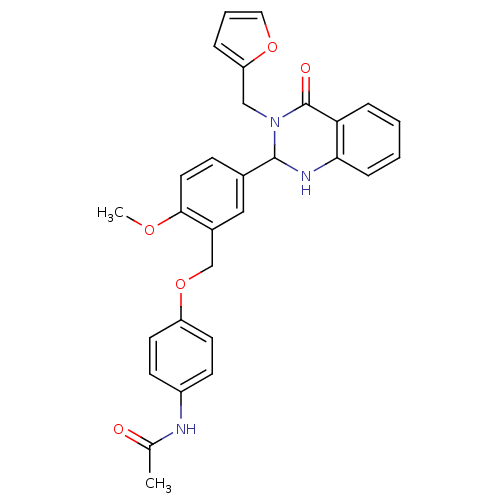 (MLS001073997 | N-[4-[5-[3-(2-furfuryl)-4-keto-1,2-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496709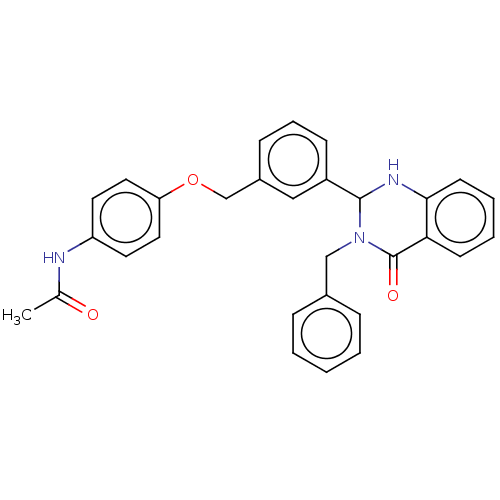 (CHEMBL1720417) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496710 (CHEMBL1563670) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496711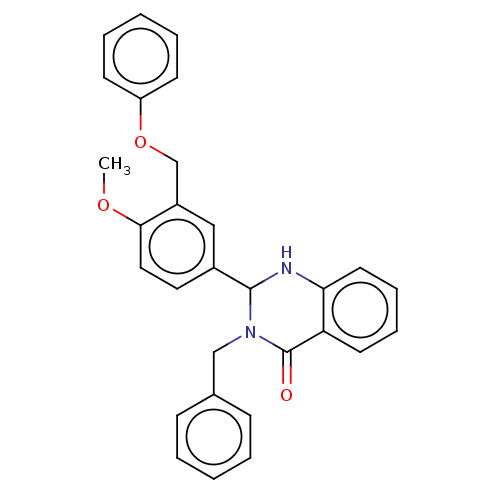 (CHEMBL1729165) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496719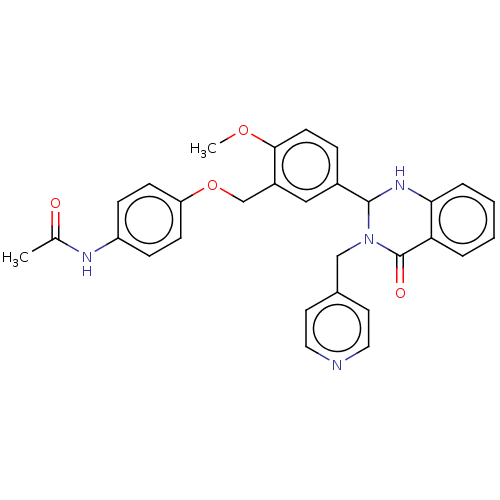 (CHEMBL1571865) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496716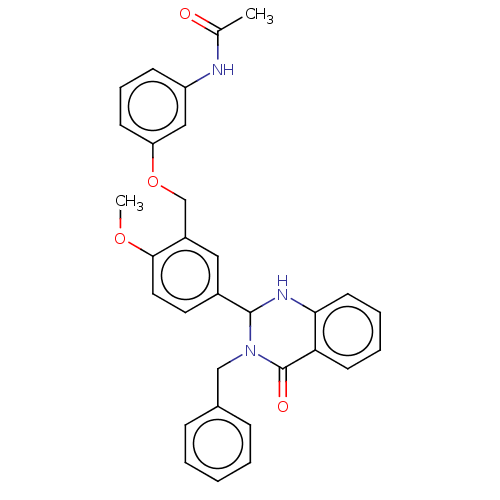 (CHEMBL1706950) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496718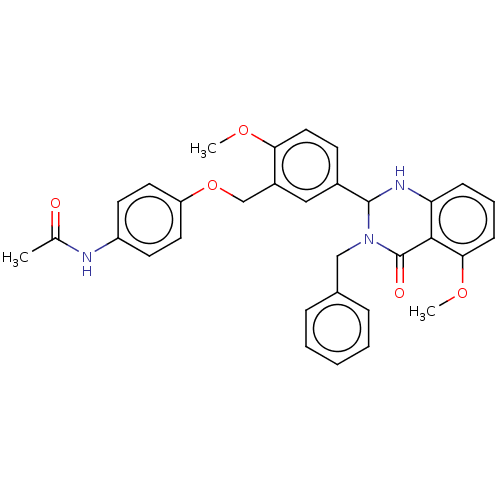 (CHEMBL1473939) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.48E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50496704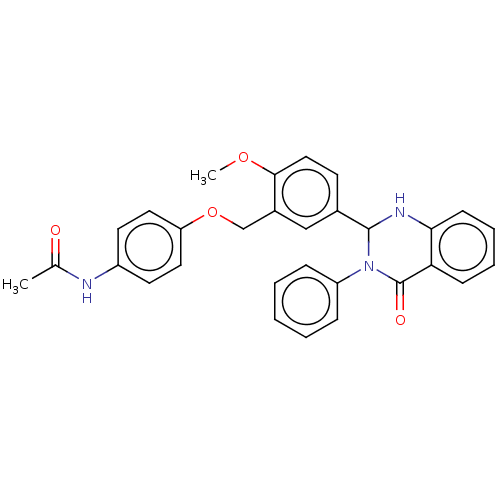 (CHEMBL1701447) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.09E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) transiently transfected HEK-EM293 cells assessed as cAMP level after 1 hr by ELISA | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50189778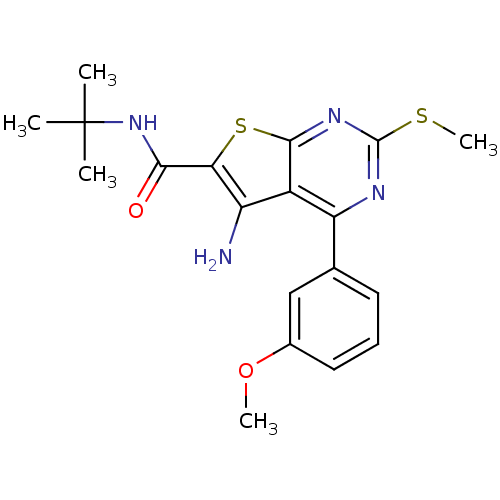 (CHEMBL211405 | N-tert-butyl-5-amino-4-(3-methoxyph...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a |
NIH Chemical Genomics Center Curated by ChEMBL | Assay Description Agonist activity at TSHR (unknown origin) | Medchemcomm 2: 1016-1020 (2011) Article DOI: 10.1039/c1md00145k BindingDB Entry DOI: 10.7270/Q2668H51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||