 Found 26 hits of ic50 for UniProtKB: P67774
Found 26 hits of ic50 for UniProtKB: P67774 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50110681

(CHEMBL430266 | Calyculin-A)Show SMILES COC[C@@H]([C@H](O)[C@H](O)C(=O)NCC[C@H](C)c1nc(\C=C\C[C@@H]2O[C@]3(C[C@@H](O)[C@@H]2C)O[C@H]([C@H](C[C@H](O)[C@H](C)[C@H](O)[C@H](C)\C=C(/C)\C(\C)=C\C=C\C(\C)=C/C#N)OC)[C@H](OP(O)(O)=O)C3(C)C)co1)N(C)C |r| Show InChI InChI=1S/C50H81N4O15P/c1-29(20-22-51)16-14-17-30(2)32(4)24-33(5)42(57)35(7)38(55)25-41(65-13)45-46(69-70(61,62)63)49(8,9)50(68-45)26-39(56)34(6)40(67-50)19-15-18-36-27-66-48(53-36)31(3)21-23-52-47(60)44(59)43(58)37(28-64-12)54(10)11/h14-18,20,24,27,31,33-35,37-46,55-59H,19,21,23,25-26,28H2,1-13H3,(H,52,60)(H2,61,62,63)/b16-14+,18-15+,29-20-,30-17+,32-24+/t31-,33+,34-,35-,37-,38-,39+,40-,41-,42+,43-,44-,45+,46-,50+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay at 50 uM |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135699
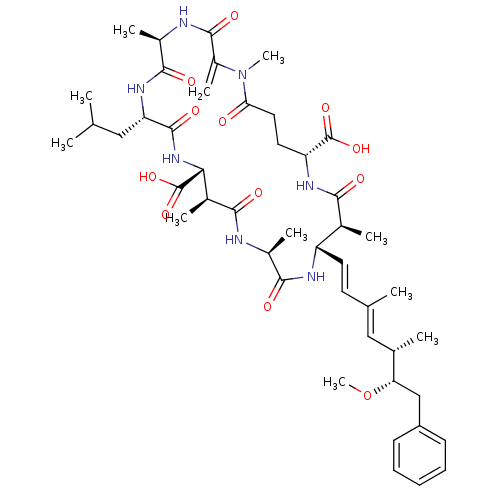
((10S,13S,18S,19S,22R)-8-Isobutyl-18-((1E,3E)-(5S,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C46H67N7O12/c1-24(2)21-35-44(60)52-38(46(63)64)28(6)40(56)47-29(7)41(57)49-33(18-17-25(3)22-26(4)36(65-11)23-32-15-13-12-14-16-32)27(5)39(55)50-34(45(61)62)19-20-37(54)53(10)31(9)43(59)48-30(8)42(58)51-35/h12-18,22,24,26-30,33-36,38H,9,19-21,23H2,1-8,10-11H3,(H,47,56)(H,48,59)(H,49,57)(H,50,55)(H,51,58)(H,52,60)(H,61,62)(H,63,64)/b18-17+,25-22+/t26-,27-,28-,29-,30+,33-,34+,35-,36-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against protein phosphatase 1 (PP1) using phosphorylase-a assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135699

((10S,13S,18S,19S,22R)-8-Isobutyl-18-((1E,3E)-(5S,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@@H]1NC(=O)[C@H](C)NC(=O)[C@@H](C)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](C)NC(=O)C(=C)N(C)C(=O)CC[C@@H](NC(=O)[C@H]1C)C(O)=O)C(O)=O Show InChI InChI=1S/C46H67N7O12/c1-24(2)21-35-44(60)52-38(46(63)64)28(6)40(56)47-29(7)41(57)49-33(18-17-25(3)22-26(4)36(65-11)23-32-15-13-12-14-16-32)27(5)39(55)50-34(45(61)62)19-20-37(54)53(10)31(9)43(59)48-30(8)42(58)51-35/h12-18,22,24,26-30,33-36,38H,9,19-21,23H2,1-8,10-11H3,(H,47,56)(H,48,59)(H,49,57)(H,50,55)(H,51,58)(H,52,60)(H,61,62)(H,63,64)/b18-17+,25-22+/t26-,27-,28-,29-,30+,33-,34+,35-,36-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50090505
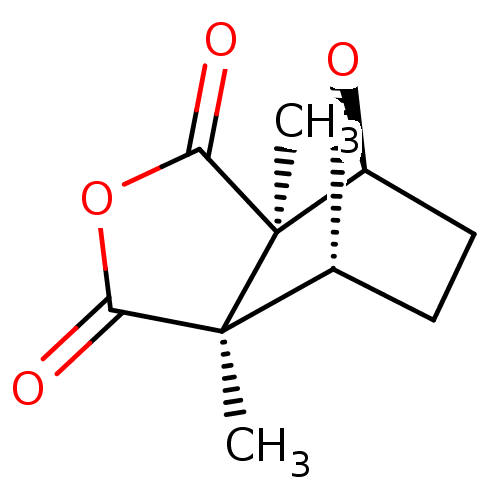
((1R,2S,6R,7S)-2,6-Dimethyl-4,10-dioxa-tricyclo[5.2...)Show SMILES C[C@]12[C@@H]3CC[C@@H](O3)[C@@]1(C)C(=O)OC2=O Show InChI InChI=1S/C10H12O4/c1-9-5-3-4-6(13-5)10(9,2)8(12)14-7(9)11/h5-6H,3-4H2,1-2H3/t5-,6+,9+,10- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at rat H3 receptor expressed in human SK-N-MC cells assessed as inhibition of forskolin-induced cAMP accumulation after 6 hrs |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135691
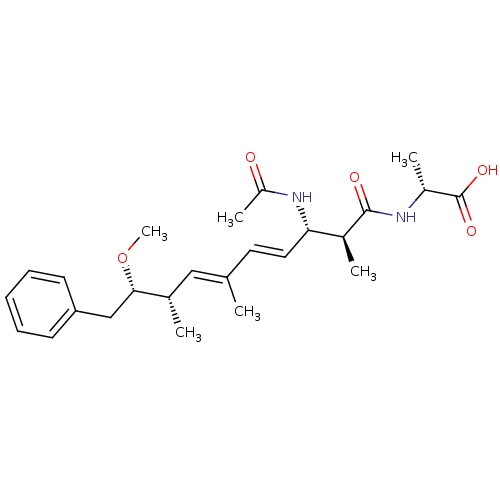
((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@H](C)C(O)=O Show InChI InChI=1S/C25H36N2O5/c1-16(14-17(2)23(32-6)15-21-10-8-7-9-11-21)12-13-22(27-20(5)28)18(3)24(29)26-19(4)25(30)31/h7-14,17-19,22-23H,15H2,1-6H3,(H,26,29)(H,27,28)(H,30,31)/b13-12+,16-14+/t17-,18-,19+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against protein phosphatase 2A (PP2A) using phosphorylase-a assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50556732
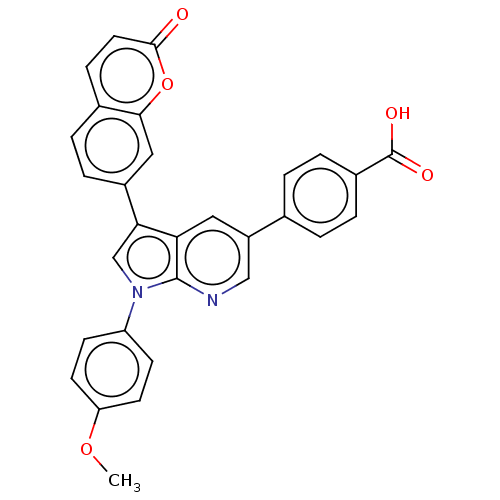
(CHEMBL4750435)Show SMILES COc1ccc(cc1)-n1cc(-c2ccc3ccc(=O)oc3c2)c2cc(cnc12)-c1ccc(cc1)C(O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PP2A alpha (unknown origin) pre-incubated for 20 mins followed by fluorescence substrate addition and measured after 120 mins by DiFMUP... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01265
BindingDB Entry DOI: 10.7270/Q24171QP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50408821
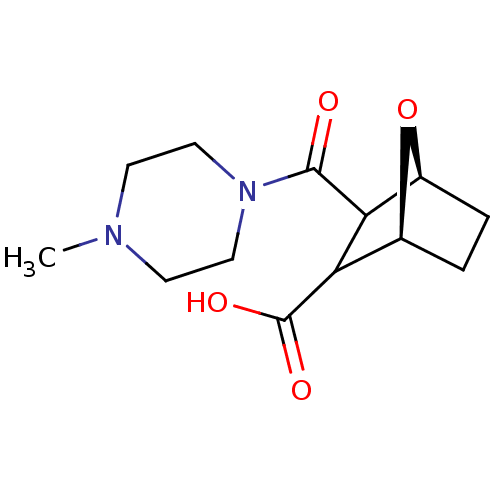
(CHEMBL5270968)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCCCCCCCCCC(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C45H74N12O8/c46-32(18-13-23-52-44(47)48)39(60)55-33(19-14-24-53-45(49)50)40(61)51-22-12-6-4-2-1-3-5-7-21-38(59)54-34(28-58)41(62)56-27-31-17-9-8-15-29(31)25-36(56)42(63)57-35-20-11-10-16-30(35)26-37(57)43(64)65/h8-9,15,17,30,32-37,58H,1-7,10-14,16,18-28,46H2,(H,51,61)(H,54,59)(H,55,60)(H,64,65)(H4,47,48,52)(H4,49,50,53)/t30-,32+,33-,34-,35-,36+,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE2 by enzyme immuno assay at 50 uM |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135697
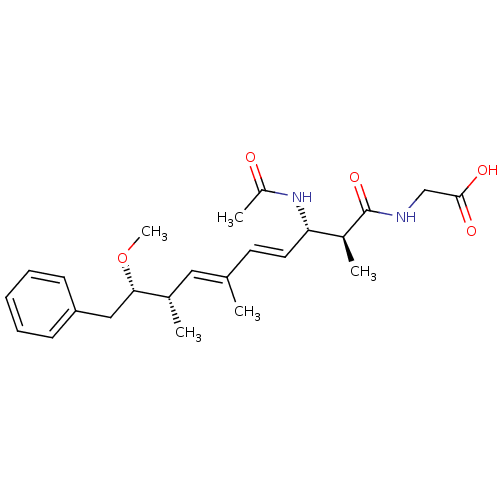
(((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-methoxy-2,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)NCC(O)=O Show InChI InChI=1S/C24H34N2O5/c1-16(13-17(2)22(31-5)14-20-9-7-6-8-10-20)11-12-21(26-19(4)27)18(3)24(30)25-15-23(28)29/h6-13,17-18,21-22H,14-15H2,1-5H3,(H,25,30)(H,26,27)(H,28,29)/b12-11+,16-13+/t17-,18-,21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against protein phosphatase 2A (PP2A) using phosphorylase-a assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50453860
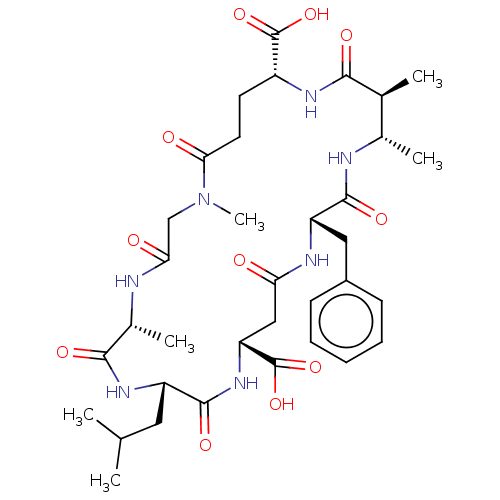
(CHEMBL4210922)Show SMILES CC(C)C[C@@H]1NC(=O)[C@@H](C)NC(=O)CN(C)C(=O)CC[C@@H](NC(=O)[C@@H](C)[C@H](C)NC(=O)[C@H](Cc2ccccc2)NC(=O)C[C@@H](NC1=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C35H51N7O11/c1-18(2)14-24-33(49)41-26(35(52)53)16-27(43)38-25(15-22-10-8-7-9-11-22)32(48)37-20(4)19(3)30(46)39-23(34(50)51)12-13-29(45)42(6)17-28(44)36-21(5)31(47)40-24/h7-11,18-21,23-26H,12-17H2,1-6H3,(H,36,44)(H,37,48)(H,38,43)(H,39,46)(H,40,47)(H,41,49)(H,50,51)(H,52,53)/t19-,20-,21+,23+,24-,25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
European Molecular Biology Laboratory (EMBL)
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PP2A catalytic subunit L309 deletion mutant using DIFMUP as substrate pretreated for 10 mins followed by substrate ad... |
Bioorg Med Chem 26: 1118-1126 (2018)
Article DOI: 10.1016/j.bmc.2017.08.040
BindingDB Entry DOI: 10.7270/Q2N58PZ3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135691

((R)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@H](C)C(O)=O Show InChI InChI=1S/C25H36N2O5/c1-16(14-17(2)23(32-6)15-21-10-8-7-9-11-21)12-13-22(27-20(5)28)18(3)24(29)26-19(4)25(30)31/h7-14,17-19,22-23H,15H2,1-6H3,(H,26,29)(H,27,28)(H,30,31)/b13-12+,16-14+/t17-,18-,19+,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135700
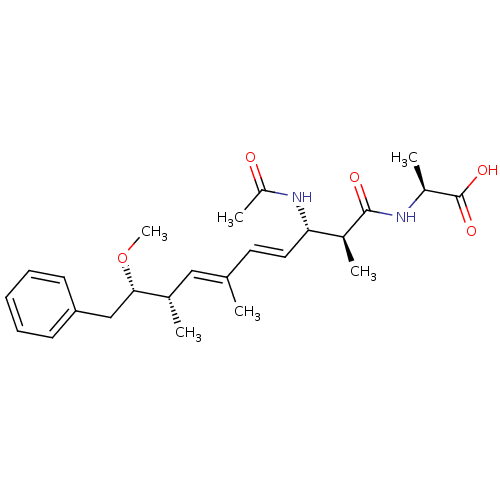
((S)-2-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N[C@@H](C)C(O)=O Show InChI InChI=1S/C25H36N2O5/c1-16(14-17(2)23(32-6)15-21-10-8-7-9-11-21)12-13-22(27-20(5)28)18(3)24(29)26-19(4)25(30)31/h7-14,17-19,22-23H,15H2,1-6H3,(H,26,29)(H,27,28)(H,30,31)/b13-12+,16-14+/t17-,18-,19-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against protein phosphatase 2A (PP2A) using phosphorylase-a assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135696
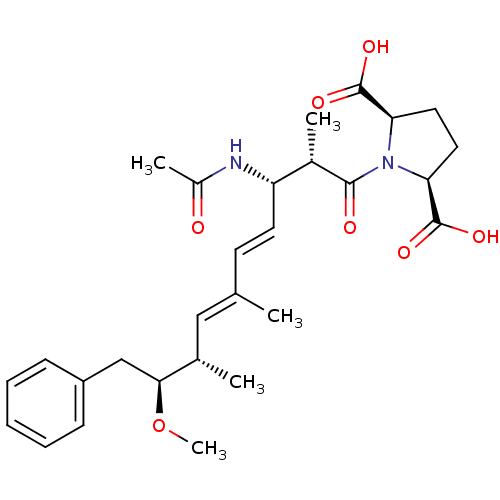
((2R,5S)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1[C@H](CC[C@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C28H38N2O7/c1-17(15-18(2)25(37-5)16-21-9-7-6-8-10-21)11-12-22(29-20(4)31)19(3)26(32)30-23(27(33)34)13-14-24(30)28(35)36/h6-12,15,18-19,22-25H,13-14,16H2,1-5H3,(H,29,31)(H,33,34)(H,35,36)/b12-11+,17-15+/t18-,19-,22-,23-,24+,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135687
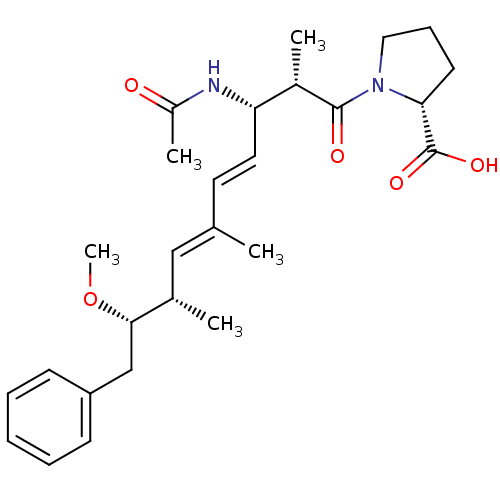
((R)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1CCC[C@@H]1C(O)=O Show InChI InChI=1S/C27H38N2O5/c1-18(16-19(2)25(34-5)17-22-10-7-6-8-11-22)13-14-23(28-21(4)30)20(3)26(31)29-15-9-12-24(29)27(32)33/h6-8,10-11,13-14,16,19-20,23-25H,9,12,15,17H2,1-5H3,(H,28,30)(H,32,33)/b14-13+,18-16+/t19-,20-,23-,24+,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135685
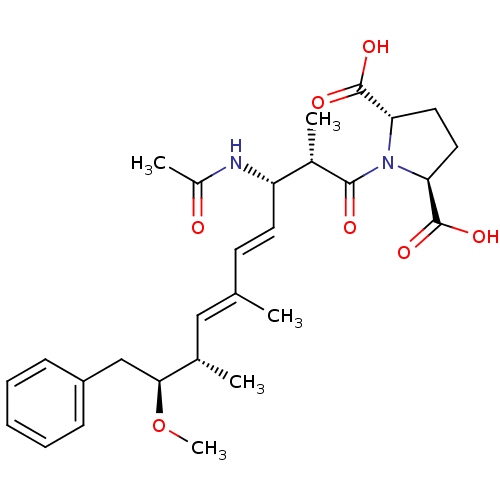
((2S,5S)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1[C@@H](CC[C@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C28H38N2O7/c1-17(15-18(2)25(37-5)16-21-9-7-6-8-10-21)11-12-22(29-20(4)31)19(3)26(32)30-23(27(33)34)13-14-24(30)28(35)36/h6-12,15,18-19,22-25H,13-14,16H2,1-5H3,(H,29,31)(H,33,34)(H,35,36)/b12-11+,17-15+/t18-,19-,22-,23-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135683
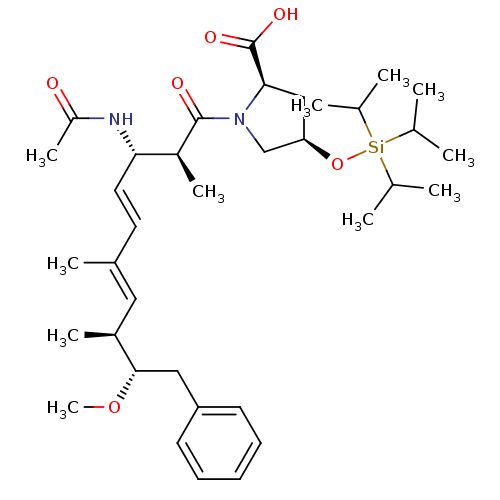
((2R,4R)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)O[Si](C(C)C)(C(C)C)C(C)C Show InChI InChI=1S/C36H58N2O6Si/c1-23(2)45(24(3)4,25(5)6)44-31-21-33(36(41)42)38(22-31)35(40)28(9)32(37-29(10)39)18-17-26(7)19-27(8)34(43-11)20-30-15-13-12-14-16-30/h12-19,23-25,27-28,31-34H,20-22H2,1-11H3,(H,37,39)(H,41,42)/b18-17+,26-19+/t27-,28-,31+,32-,33+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135686
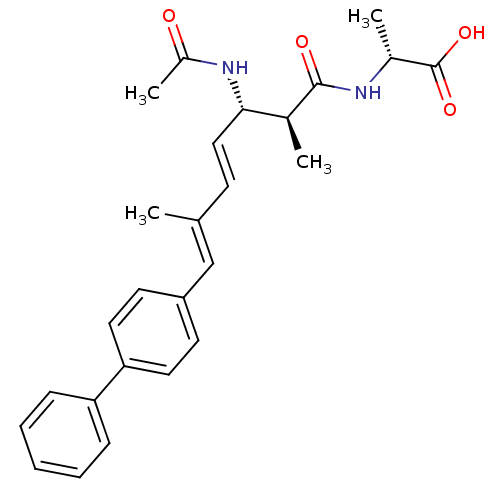
((R)-2-((4E,6E)-(2S,3S)-3-Acetylamino-7-biphenyl-4-...)Show SMILES C[C@@H](NC(=O)[C@@H](C)[C@@H](NC(C)=O)\C=C\C(\C)=C\c1ccc(cc1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C26H30N2O4/c1-17(16-21-11-13-23(14-12-21)22-8-6-5-7-9-22)10-15-24(28-20(4)29)18(2)25(30)27-19(3)26(31)32/h5-16,18-19,24H,1-4H3,(H,27,30)(H,28,29)(H,31,32)/b15-10+,17-16+/t18-,19+,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135689
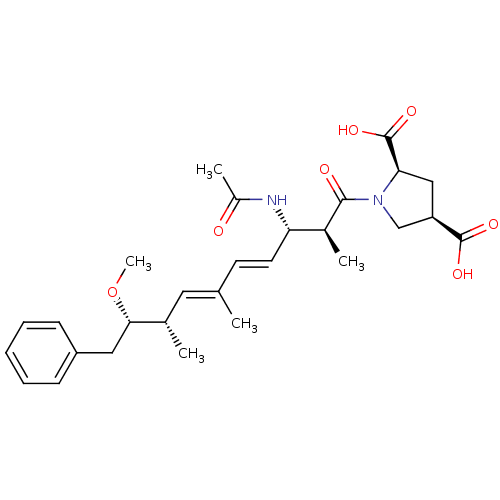
((2R,4R)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C28H38N2O7/c1-17(13-18(2)25(37-5)14-21-9-7-6-8-10-21)11-12-23(29-20(4)31)19(3)26(32)30-16-22(27(33)34)15-24(30)28(35)36/h6-13,18-19,22-25H,14-16H2,1-5H3,(H,29,31)(H,33,34)(H,35,36)/b12-11+,17-13+/t18-,19-,22+,23-,24+,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135684
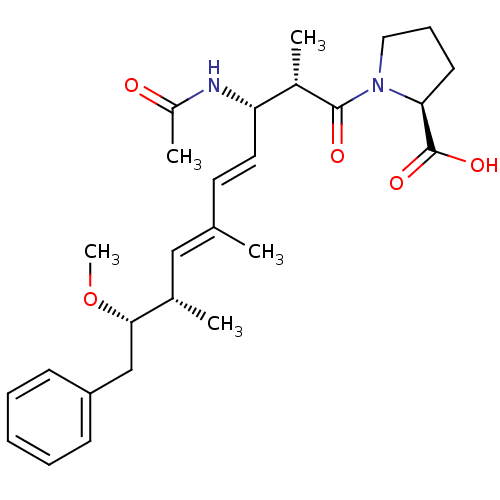
((S)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C27H38N2O5/c1-18(16-19(2)25(34-5)17-22-10-7-6-8-11-22)13-14-23(28-21(4)30)20(3)26(31)29-15-9-12-24(29)27(32)33/h6-8,10-11,13-14,16,19-20,23-25H,9,12,15,17H2,1-5H3,(H,28,30)(H,32,33)/b14-13+,18-16+/t19-,20-,23-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135688
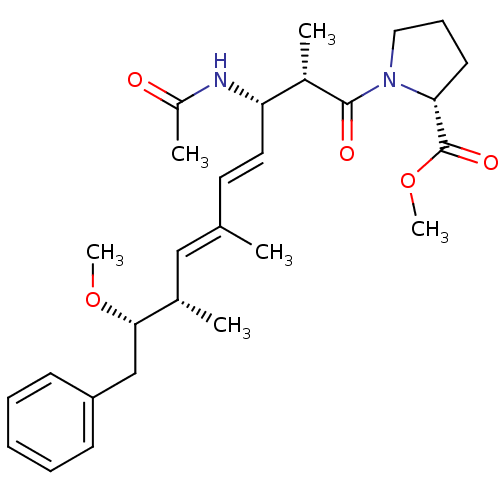
((R)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1CCC[C@@H]1C(=O)OC Show InChI InChI=1S/C28H40N2O5/c1-19(17-20(2)26(34-5)18-23-11-8-7-9-12-23)14-15-24(29-22(4)31)21(3)27(32)30-16-10-13-25(30)28(33)35-6/h7-9,11-12,14-15,17,20-21,24-26H,10,13,16,18H2,1-6H3,(H,29,31)/b15-14+,19-17+/t20-,21-,24-,25+,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135682
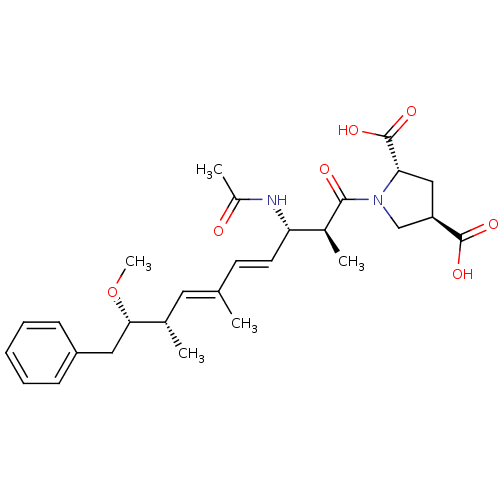
((2S,4R)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1C[C@@H](C[C@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C28H38N2O7/c1-17(13-18(2)25(37-5)14-21-9-7-6-8-10-21)11-12-23(29-20(4)31)19(3)26(32)30-16-22(27(33)34)15-24(30)28(35)36/h6-13,18-19,22-25H,14-16H2,1-5H3,(H,29,31)(H,33,34)(H,35,36)/b12-11+,17-13+/t18-,19-,22+,23-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135693
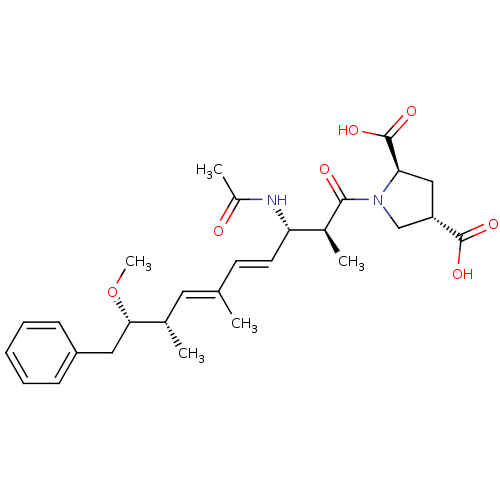
((2R,4S)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-m...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1C[C@H](C[C@@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C28H38N2O7/c1-17(13-18(2)25(37-5)14-21-9-7-6-8-10-21)11-12-23(29-20(4)31)19(3)26(32)30-16-22(27(33)34)15-24(30)28(35)36/h6-13,18-19,22-25H,14-16H2,1-5H3,(H,29,31)(H,33,34)(H,35,36)/b12-11+,17-13+/t18-,19-,22-,23-,24+,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135690
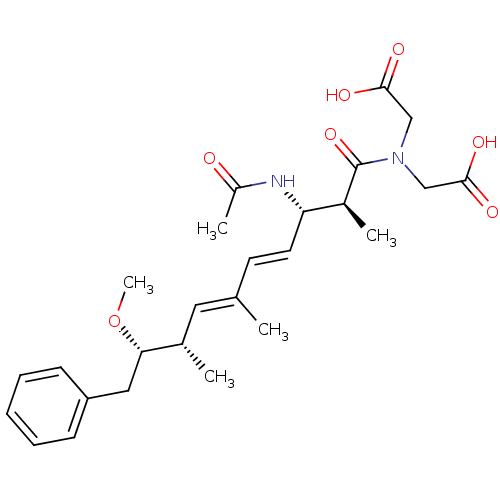
(CHEMBL98661 | [((4E,6E)-(2S,3S,8S,9S)-3-Acetylamin...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N(CC(O)=O)CC(O)=O Show InChI InChI=1S/C26H36N2O7/c1-17(13-18(2)23(35-5)14-21-9-7-6-8-10-21)11-12-22(27-20(4)29)19(3)26(34)28(15-24(30)31)16-25(32)33/h6-13,18-19,22-23H,14-16H2,1-5H3,(H,27,29)(H,30,31)(H,32,33)/b12-11+,17-13+/t18-,19-,22-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135698
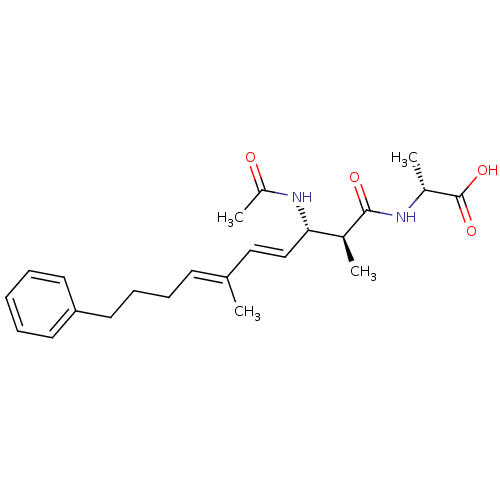
((R)-2-((4E,6E)-(2S,3S)-3-Acetylamino-2,6-dimethyl-...)Show SMILES C[C@@H](NC(=O)[C@@H](C)[C@@H](NC(C)=O)\C=C\C(\C)=C\CCCc1ccccc1)C(O)=O Show InChI InChI=1S/C23H32N2O4/c1-16(10-8-9-13-20-11-6-5-7-12-20)14-15-21(25-19(4)26)17(2)22(27)24-18(3)23(28)29/h5-7,10-12,14-15,17-18,21H,8-9,13H2,1-4H3,(H,24,27)(H,25,26)(H,28,29)/b15-14+,16-10+/t17-,18+,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135694
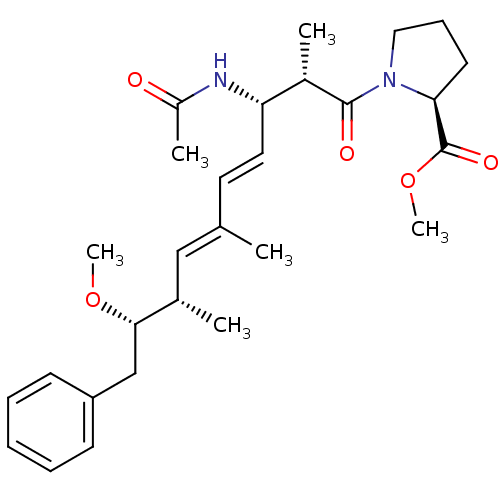
((S)-1-((4E,6E)-(2S,3S,8S,9S)-3-Acetylamino-9-metho...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1CCC[C@H]1C(=O)OC Show InChI InChI=1S/C28H40N2O5/c1-19(17-20(2)26(34-5)18-23-11-8-7-9-12-23)14-15-24(29-22(4)31)21(3)27(32)30-16-10-13-25(30)28(33)35-6/h7-9,11-12,14-15,17,20-21,24-26H,10,13,16,18H2,1-6H3,(H,29,31)/b15-14+,19-17+/t20-,21-,24-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135692
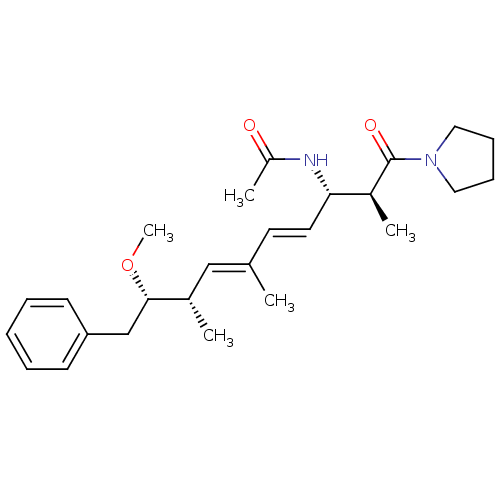
(CHEMBL100880 | N-[(2E,4E)-(1S,6S,7S)-7-Methoxy-4,6...)Show SMILES CO[C@@H](Cc1ccccc1)[C@@H](C)\C=C(/C)\C=C\[C@H](NC(C)=O)[C@H](C)C(=O)N1CCCC1 Show InChI InChI=1S/C26H38N2O3/c1-19(17-20(2)25(31-5)18-23-11-7-6-8-12-23)13-14-24(27-22(4)29)21(3)26(30)28-15-9-10-16-28/h6-8,11-14,17,20-21,24-25H,9-10,15-16,18H2,1-5H3,(H,27,29)/b14-13+,19-17+/t20-,21-,24-,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein phosphatase 2A catalytic subunit alpha isoform
(Bos taurus) | BDBM50135695
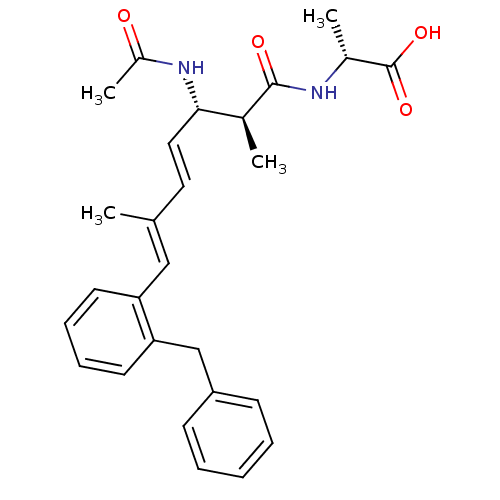
((R)-2-[(4E,6E)-(2S,3S)-3-Acetylamino-7-(2-benzyl-p...)Show SMILES C[C@@H](NC(=O)[C@@H](C)[C@@H](NC(C)=O)\C=C\C(\C)=C\c1ccccc1Cc1ccccc1)C(O)=O Show InChI InChI=1S/C27H32N2O4/c1-18(16-23-12-8-9-13-24(23)17-22-10-6-5-7-11-22)14-15-25(29-21(4)30)19(2)26(31)28-20(3)27(32)33/h5-16,19-20,25H,17H2,1-4H3,(H,28,31)(H,29,30)(H,32,33)/b15-14+,18-16+/t19-,20+,25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California at Irvine
Curated by ChEMBL
| Assay Description
Inhibitory concentration against protein phosphatase 2A (PP2A) using pNPP assay |
Bioorg Med Chem Lett 13: 2907-11 (2003)
BindingDB Entry DOI: 10.7270/Q2SF2VKZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data