 Found 292 hits of ic50 for UniProtKB: P0A725
Found 292 hits of ic50 for UniProtKB: P0A725 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491052
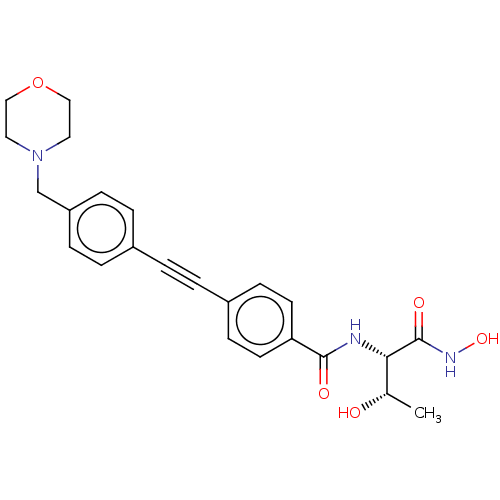
(CHEMBL2377693)Show SMILES C[C@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501754

(CHEMBL4071396)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H33N3O4/c1-19(31)26(27(32)29-33)28-25-4-2-3-23-17-21(11-12-24(23)25)8-5-20-6-9-22(10-7-20)18-30-13-15-34-16-14-30/h6-7,9-12,17,19,25-26,28,31,33H,2-4,13-16,18H2,1H3,(H,29,32)/t19-,25?,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483398

(CHEMBL1668460)Show SMILES CC(C)(O)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C24H28N4O4/c1-24(2,31)21(22(29)26-32)25-23(30)28-16-14-27(15-17-28)20-12-10-19(11-13-20)9-8-18-6-4-3-5-7-18/h3-7,10-13,21,31-32H,14-17H2,1-2H3,(H,25,30)(H,26,29)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483411
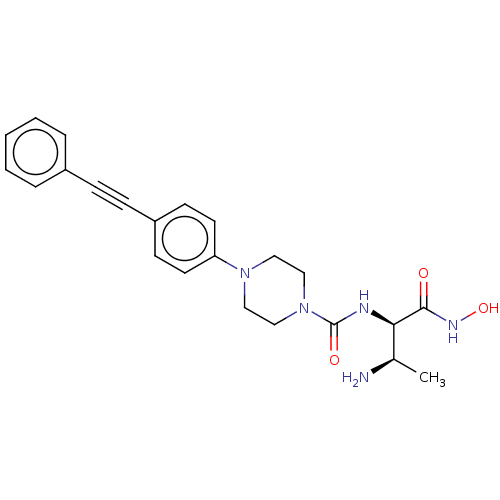
(CHEMBL1668464)Show SMILES C[C@@H](N)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C23H27N5O3/c1-17(24)21(22(29)26-31)25-23(30)28-15-13-27(14-16-28)20-11-9-19(10-12-20)8-7-18-5-3-2-4-6-18/h2-6,9-12,17,21,31H,13-16,24H2,1H3,(H,25,30)(H,26,29)/t17-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483411

(CHEMBL1668464)Show SMILES C[C@@H](N)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C23H27N5O3/c1-17(24)21(22(29)26-31)25-23(30)28-15-13-27(14-16-28)20-11-9-19(10-12-20)8-7-18-5-3-2-4-6-18/h2-6,9-12,17,21,31H,13-16,24H2,1H3,(H,25,30)(H,26,29)/t17-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501779
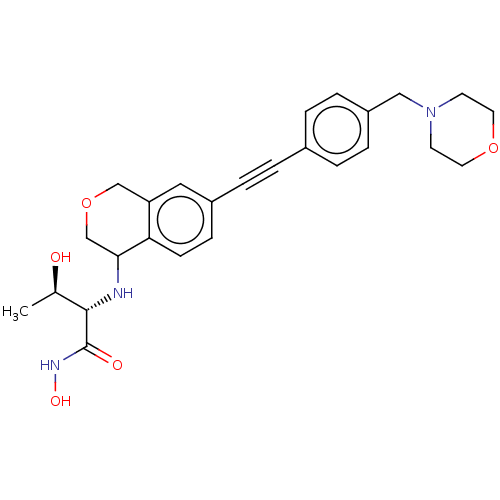
(CHEMBL4102207)Show SMILES C[C@@H](O)[C@H](NC1COCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O5/c1-18(30)25(26(31)28-32)27-24-17-34-16-22-14-20(8-9-23(22)24)5-2-19-3-6-21(7-4-19)15-29-10-12-33-13-11-29/h3-4,6-9,14,18,24-25,27,30,32H,10-13,15-17H2,1H3,(H,28,31)/t18-,24?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501764
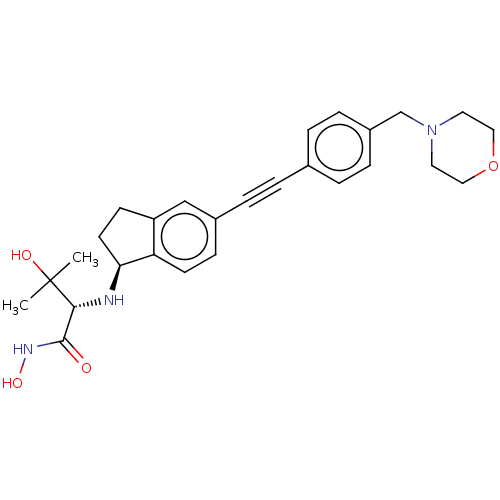
(CHEMBL4061199)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)O |r| Show InChI InChI=1S/C27H33N3O4/c1-27(2,32)25(26(31)29-33)28-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-30-13-15-34-16-14-30/h4-5,7-9,11,17,24-25,28,32-33H,10,12-16,18H2,1-2H3,(H,29,31)/t24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501782

(CHEMBL4087371)Show SMILES C[C@@H](O)[C@H](NC1CCCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H24N2O3/c1-15(25)21(22(26)24-27)23-20-9-5-8-18-14-17(12-13-19(18)20)11-10-16-6-3-2-4-7-16/h2-4,6-7,12-15,20-21,23,25,27H,5,8-9H2,1H3,(H,24,26)/t15-,20?,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491048
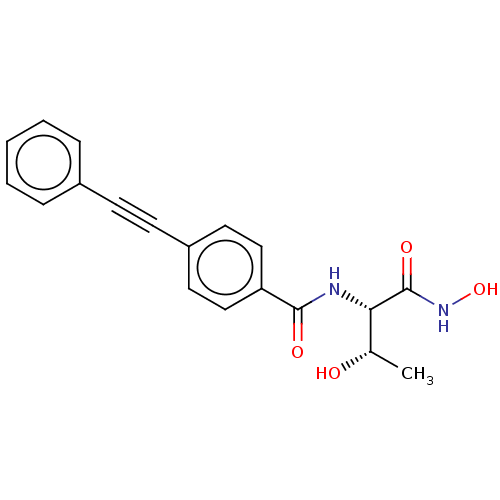
(CHEMBL2377694)Show SMILES C[C@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C19H18N2O4/c1-13(22)17(19(24)21-25)20-18(23)16-11-9-15(10-12-16)8-7-14-5-3-2-4-6-14/h2-6,9-13,17,22,25H,1H3,(H,20,23)(H,21,24)/t13-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501762

(CHEMBL4081478)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2C)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H28N4O3/c1-17(31)25(26(32)29-33)28-24-12-10-22-15-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)16-30-14-13-27-18(30)2/h4-5,7-9,11,13-15,17,24-25,28,31,33H,10,12,16H2,1-2H3,(H,29,32)/t17-,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501755
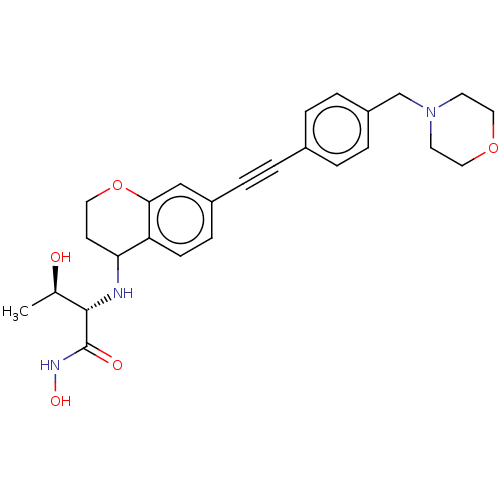
(CHEMBL4077947)Show SMILES C[C@@H](O)[C@H](NC1CCOc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O5/c1-18(30)25(26(31)28-32)27-23-10-13-34-24-16-20(8-9-22(23)24)5-2-19-3-6-21(7-4-19)17-29-11-14-33-15-12-29/h3-4,6-9,16,18,23,25,27,30,32H,10-15,17H2,1H3,(H,28,31)/t18-,23?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491047
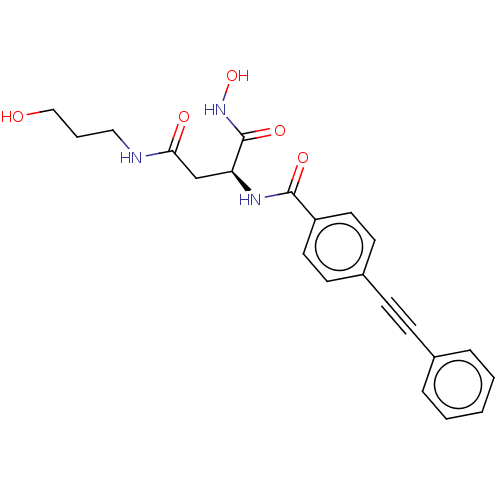
(CHEMBL2377569)Show SMILES OCCCNC(=O)C[C@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H23N3O5/c26-14-4-13-23-20(27)15-19(22(29)25-30)24-21(28)18-11-9-17(10-12-18)8-7-16-5-2-1-3-6-16/h1-3,5-6,9-12,19,26,30H,4,13-15H2,(H,23,27)(H,24,28)(H,25,29)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491046
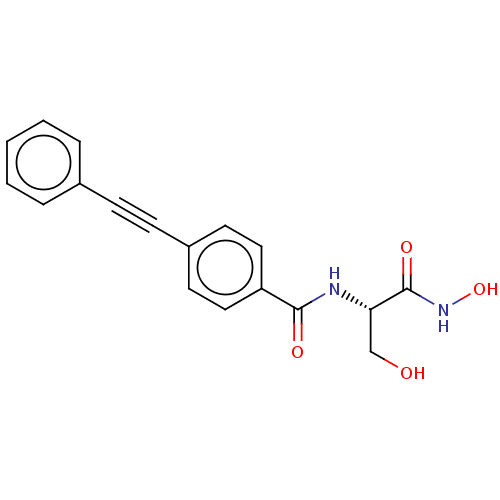
(CHEMBL2377699)Show SMILES OC[C@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C18H16N2O4/c21-12-16(18(23)20-24)19-17(22)15-10-8-14(9-11-15)7-6-13-4-2-1-3-5-13/h1-5,8-11,16,21,24H,12H2,(H,19,22)(H,20,23)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491056

(CHEMBL2377567)Show SMILES ONC(=O)[C@H](Cc1cnc[nH]1)NC(=O)c1ccc(cc1)C#Cc1ccccc1 |r| Show InChI InChI=1S/C21H18N4O3/c26-20(24-19(21(27)25-28)12-18-13-22-14-23-18)17-10-8-16(9-11-17)7-6-15-4-2-1-3-5-15/h1-5,8-11,13-14,19,28H,12H2,(H,22,23)(H,24,26)(H,25,27)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491051
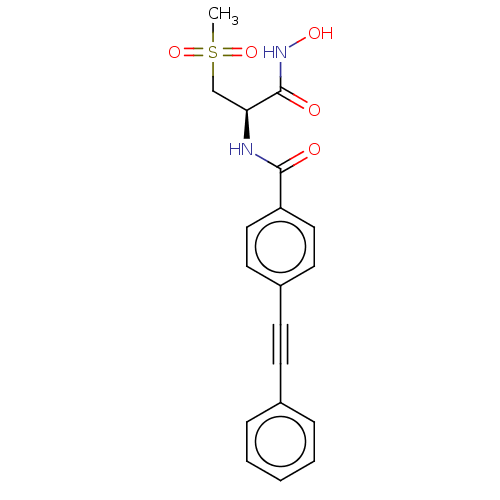
(CHEMBL2377703)Show SMILES CS(=O)(=O)C[C@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C19H18N2O5S/c1-27(25,26)13-17(19(23)21-24)20-18(22)16-11-9-15(10-12-16)8-7-14-5-3-2-4-6-14/h2-6,9-12,17,24H,13H2,1H3,(H,20,22)(H,21,23)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491045
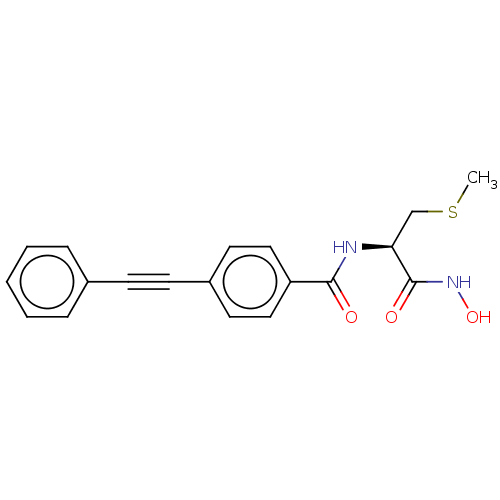
(CHEMBL2377702)Show SMILES CSC[C@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C19H18N2O3S/c1-25-13-17(19(23)21-24)20-18(22)16-11-9-15(10-12-16)8-7-14-5-3-2-4-6-14/h2-6,9-12,17,24H,13H2,1H3,(H,20,22)(H,21,23)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501752
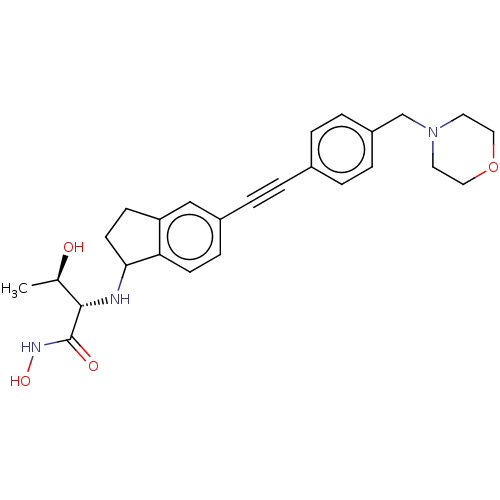
(CHEMBL4095059)Show SMILES C[C@@H](O)[C@H](NC1CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24?,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491044
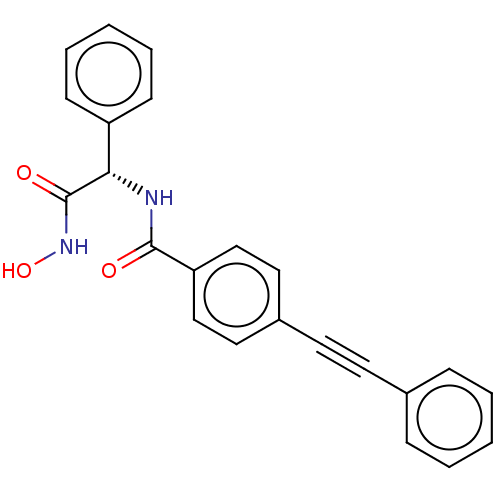
(CHEMBL2377704)Show SMILES ONC(=O)[C@@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C23H18N2O3/c26-22(24-21(23(27)25-28)19-9-5-2-6-10-19)20-15-13-18(14-16-20)12-11-17-7-3-1-4-8-17/h1-10,13-16,21,28H,(H,24,26)(H,25,27)/t21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501753

(CHEMBL4098438)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501780
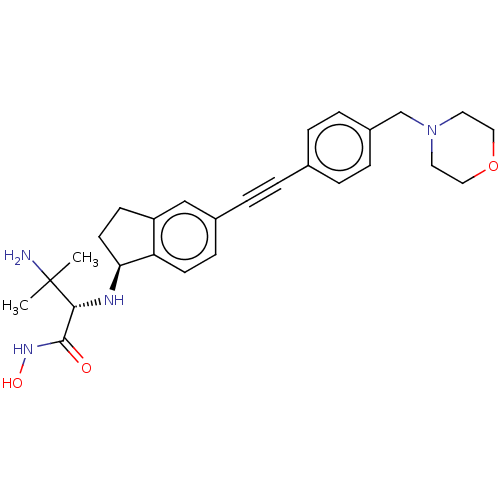
(CHEMBL4083988)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H34N4O3/c1-27(2,28)25(26(32)30-33)29-24-12-10-22-17-20(9-11-23(22)24)6-3-19-4-7-21(8-5-19)18-31-13-15-34-16-14-31/h4-5,7-9,11,17,24-25,29,33H,10,12-16,18,28H2,1-2H3,(H,30,32)/t24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501756
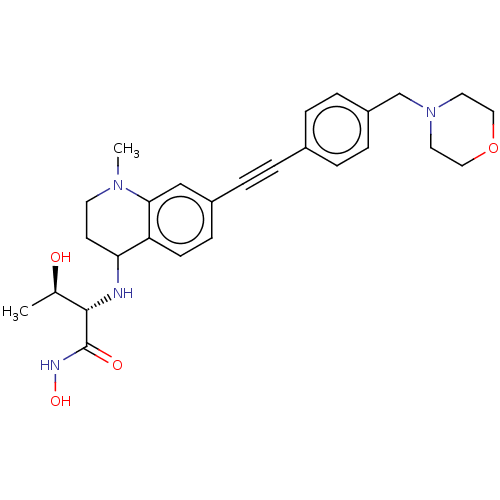
(CHEMBL4066982)Show SMILES C[C@@H](O)[C@H](NC1CCN(C)c2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H34N4O4/c1-19(32)26(27(33)29-34)28-24-11-12-30(2)25-17-21(9-10-23(24)25)6-3-20-4-7-22(8-5-20)18-31-13-15-35-16-14-31/h4-5,7-10,17,19,24,26,28,32,34H,11-16,18H2,1-2H3,(H,29,33)/t19-,24?,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483376

(CHEMBL1668422 | CHR-12)Show SMILES C[C@@H](O)[C@@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C19H18N2O4/c1-13(22)17(19(24)21-25)20-18(23)16-11-9-15(10-12-16)8-7-14-5-3-2-4-6-14/h2-6,9-13,17,22,25H,1H3,(H,20,23)(H,21,24)/t13-,17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501768
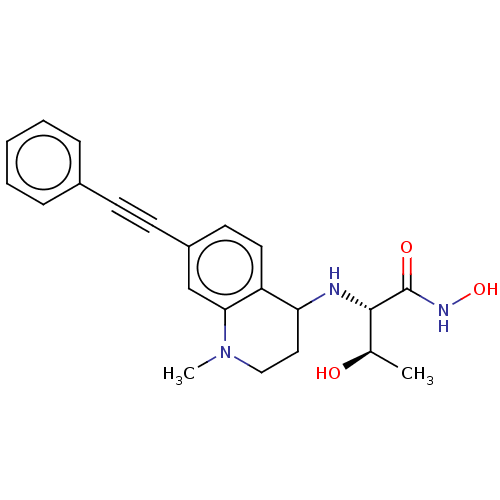
(CHEMBL4102818)Show SMILES C[C@@H](O)[C@H](NC1CCN(C)c2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H25N3O3/c1-15(26)21(22(27)24-28)23-19-12-13-25(2)20-14-17(10-11-18(19)20)9-8-16-6-4-3-5-7-16/h3-7,10-11,14-15,19,21,23,26,28H,12-13H2,1-2H3,(H,24,27)/t15-,19?,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501778

(CHEMBL4098674)Show SMILES C[C@@H](O)[C@H](NC1COCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O4/c1-14(24)20(21(25)23-26)22-19-13-27-12-17-11-16(9-10-18(17)19)8-7-15-5-3-2-4-6-15/h2-6,9-11,14,19-20,22,24,26H,12-13H2,1H3,(H,23,25)/t14-,19?,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483384

(CHEMBL1668437)Show SMILES C[C@@H](O)[C@@H](NC(=O)N1CCC(CC1)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O4/c1-17(28)22(23(29)26-31)25-24(30)27-15-13-21(14-16-27)20-11-9-19(10-12-20)8-7-18-5-3-2-4-6-18/h2-6,9-12,17,21-22,28,31H,13-16H2,1H3,(H,25,30)(H,26,29)/t17-,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483385

(CHEMBL1668438)Show SMILES C[C@@H](O)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C23H26N4O4/c1-17(28)21(22(29)25-31)24-23(30)27-15-13-26(14-16-27)20-11-9-19(10-12-20)8-7-18-5-3-2-4-6-18/h2-6,9-12,17,21,28,31H,13-16H2,1H3,(H,24,30)(H,25,29)/t17-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483402
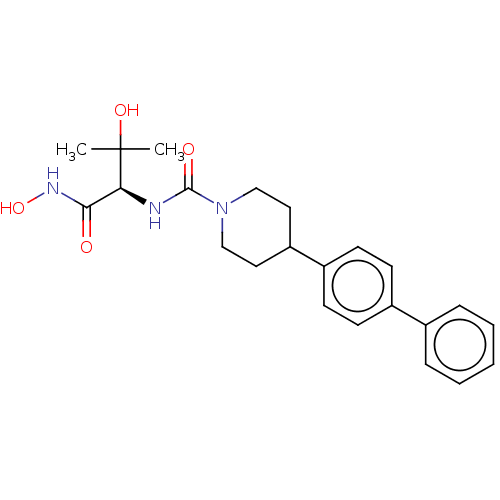
(CHEMBL1668461)Show SMILES CC(C)(O)[C@@H](NC(=O)N1CCC(CC1)c1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C23H29N3O4/c1-23(2,29)20(21(27)25-30)24-22(28)26-14-12-19(13-15-26)18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-11,19-20,29-30H,12-15H2,1-2H3,(H,24,28)(H,25,27)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate measured after 60 mins by fluorescence analysis |
ACS Med Chem Lett 7: 623-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00057
BindingDB Entry DOI: 10.7270/Q2DV1NWV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501783
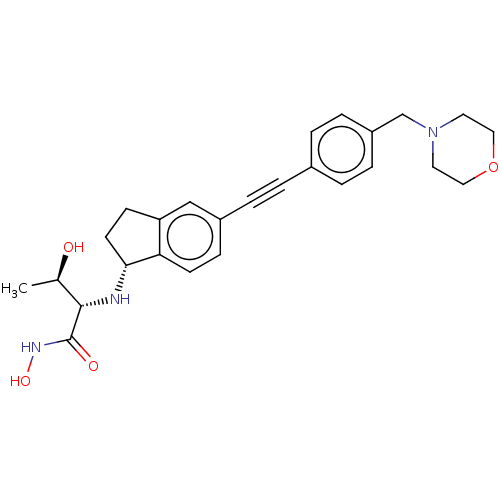
(CHEMBL4064896)Show SMILES [H][C@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H31N3O4/c1-18(30)25(26(31)28-32)27-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-29-12-14-33-15-13-29/h3-4,6-8,10,16,18,24-25,27,30,32H,9,11-15,17H2,1H3,(H,28,31)/t18-,24-,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501767

(CHEMBL4091763)Show SMILES CC(C)(N)[C@H](NC1COCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CO)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H31N5O4/c1-27(2,28)25(26(34)31-35)30-23-17-36-16-21-13-19(9-10-22(21)23)6-3-18-4-7-20(8-5-18)14-32-12-11-29-24(32)15-33/h4-5,7-13,23,25,30,33,35H,14-17,28H2,1-2H3,(H,31,34)/t23?,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483397

(CHEMBL1668458)Show SMILES C[C@@H](O)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)C#Cc1ccc(cc1)N1CCOCC1)C(=O)NO |r| Show InChI InChI=1S/C27H33N5O5/c1-20(33)25(26(34)29-36)28-27(35)32-14-12-30(13-15-32)23-8-4-21(5-9-23)2-3-22-6-10-24(11-7-22)31-16-18-37-19-17-31/h4-11,20,25,33,36H,12-19H2,1H3,(H,28,35)(H,29,34)/t20-,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate after 60 mins by OPA reagent based fluorescence assay |
Bioorg Med Chem Lett 27: 1045-1049 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.059
BindingDB Entry DOI: 10.7270/Q2MC9313 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491050

(CHEMBL2377698)Show InChI InChI=1S/C20H20N2O3/c1-14(2)18(20(24)22-25)21-19(23)17-12-10-16(11-13-17)9-8-15-6-4-3-5-7-15/h3-7,10-14,18,25H,1-2H3,(H,21,23)(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491055
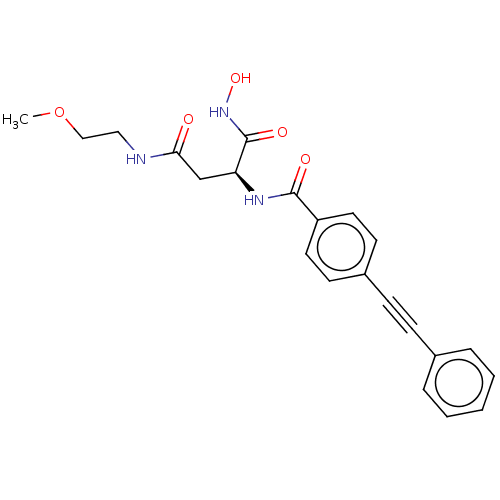
(CHEMBL2377570)Show SMILES COCCNC(=O)C[C@H](NC(=O)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H23N3O5/c1-30-14-13-23-20(26)15-19(22(28)25-29)24-21(27)18-11-9-17(10-12-18)8-7-16-5-3-2-4-6-16/h2-6,9-12,19,29H,13-15H2,1H3,(H,23,26)(H,24,27)(H,25,28)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483389
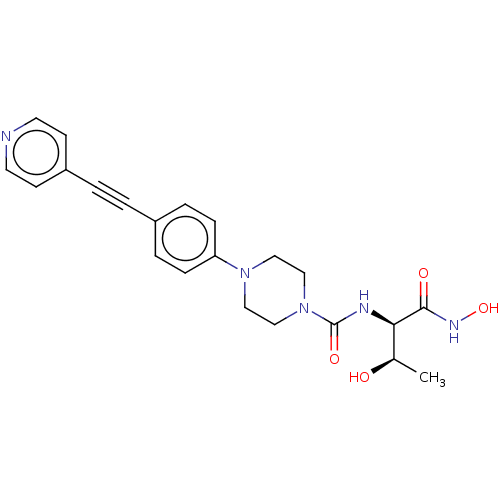
(CHEMBL1668445)Show SMILES C[C@@H](O)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)C#Cc1ccncc1)C(=O)NO |r| Show InChI InChI=1S/C22H25N5O4/c1-16(28)20(21(29)25-31)24-22(30)27-14-12-26(13-15-27)19-6-4-17(5-7-19)2-3-18-8-10-23-11-9-18/h4-11,16,20,28,31H,12-15H2,1H3,(H,24,30)(H,25,29)/t16-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491043
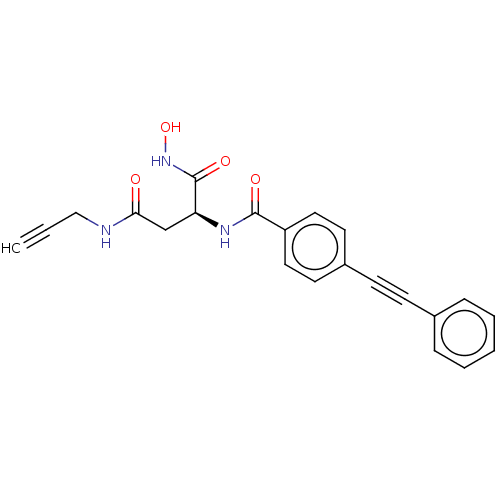
(CHEMBL2377571)Show SMILES ONC(=O)[C@H](CC(=O)NCC#C)NC(=O)c1ccc(cc1)C#Cc1ccccc1 |r| Show InChI InChI=1S/C22H19N3O4/c1-2-14-23-20(26)15-19(22(28)25-29)24-21(27)18-12-10-17(11-13-18)9-8-16-6-4-3-5-7-16/h1,3-7,10-13,19,29H,14-15H2,(H,23,26)(H,24,27)(H,25,28)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50491058

(CHEMBL2377690)Show SMILES ONC(=O)C1(CCS(=O)(=O)CC1)NC(=O)c1ccc(cc1)C#Cc1ccccc1 Show InChI InChI=1S/C21H20N2O5S/c24-19(22-21(20(25)23-26)12-14-29(27,28)15-13-21)18-10-8-17(9-11-18)7-6-16-4-2-1-3-5-16/h1-5,8-11,26H,12-15H2,(H,22,24)(H,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli str. K-12 substr. MG1655 LpxC using UDP-3-O-(R-3-hydroxydecanoyl)-N-acetylglucosamine as substrate preincubated for 20... |
Bioorg Med Chem Lett 23: 2362-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.02.055
BindingDB Entry DOI: 10.7270/Q2F76GGD |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483392

(CHEMBL1668450)Show SMILES C[C@@H](O)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)C#Cc1ccc(O)cc1)C(=O)NO |r| Show InChI InChI=1S/C23H26N4O5/c1-16(28)21(22(30)25-32)24-23(31)27-14-12-26(13-15-27)19-8-4-17(5-9-19)2-3-18-6-10-20(29)11-7-18/h4-11,16,21,28-29,32H,12-15H2,1H3,(H,24,31)(H,25,30)/t16-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501770
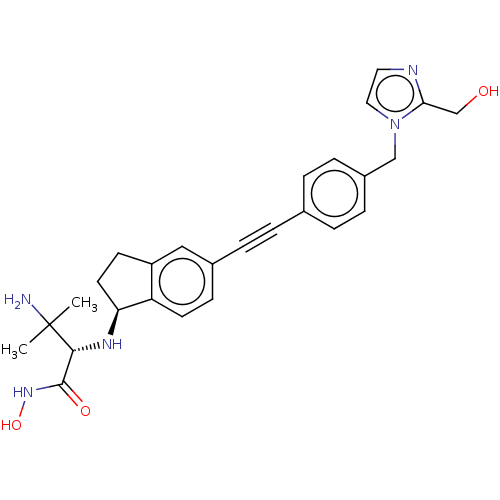
(CHEMBL4092719)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CO)cc1)N[C@H](C(=O)NO)C(C)(C)N |r| Show InChI InChI=1S/C27H31N5O3/c1-27(2,28)25(26(34)31-35)30-23-12-10-21-15-19(9-11-22(21)23)6-3-18-4-7-20(8-5-18)16-32-14-13-29-24(32)17-33/h4-5,7-9,11,13-15,23,25,30,33,35H,10,12,16-17,28H2,1-2H3,(H,31,34)/t23-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM166414

(US9073821, 402)Show SMILES CNC(=O)[C@](C)(N(C)C(=O)c1ccc(cc1)-c1ccc2n(C)ccc2c1)C(=O)NO |r| Show InChI InChI=1S/C22H24N4O4/c1-22(20(28)23-2,21(29)24-30)26(4)19(27)15-7-5-14(6-8-15)16-9-10-18-17(13-16)11-12-25(18)3/h5-13,30H,1-4H3,(H,23,28)(H,24,29)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 6.5 | 25 |
TAISHO PHARMACEUTICAL CO., LTD; TOYAMA CHEMICAL CO., LTD.
US Patent
| Assay Description
To assay the activity of E. coli LpxC enzyme, LpxC was reacted with its substrate UDP-3-O-(R-3-hydroxytetradecanoyl)-N-acetylglucosamine and the amou... |
US Patent US9073821 (2015)
BindingDB Entry DOI: 10.7270/Q2ZG6R0H |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
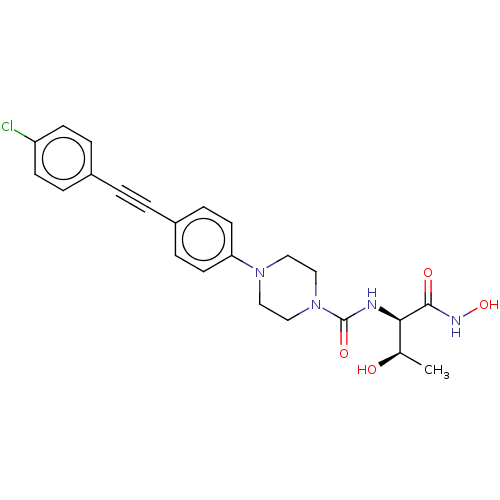
(Escherichia coli) | BDBM50483412
(CHEMBL1668452)Show SMILES C[C@@H](O)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)C#Cc1ccc(Cl)cc1)C(=O)NO |r| Show InChI InChI=1S/C23H25ClN4O4/c1-16(29)21(22(30)26-32)25-23(31)28-14-12-27(13-15-28)20-10-6-18(7-11-20)3-2-17-4-8-19(24)9-5-17/h4-11,16,21,29,32H,12-15H2,1H3,(H,25,31)(H,26,30)/t16-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
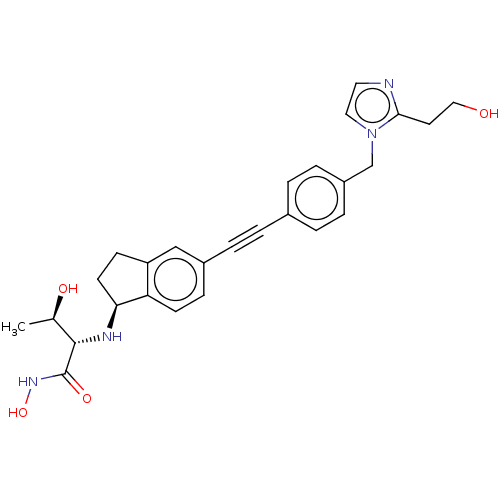
(Escherichia coli) | BDBM50501763
(CHEMBL4099505)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CCO)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C27H30N4O4/c1-18(33)26(27(34)30-35)29-24-11-9-22-16-20(8-10-23(22)24)5-2-19-3-6-21(7-4-19)17-31-14-13-28-25(31)12-15-32/h3-4,6-8,10,13-14,16,18,24,26,29,32-33,35H,9,11-12,15,17H2,1H3,(H,30,34)/t18-,24+,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501761

(CHEMBL4068010)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(CN2CCC(CC2)OC)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C28H35N3O4/c1-19(32)27(28(33)30-34)29-26-12-10-23-17-21(9-11-25(23)26)6-3-20-4-7-22(8-5-20)18-31-15-13-24(35-2)14-16-31/h4-5,7-9,11,17,19,24,26-27,29,32,34H,10,12-16,18H2,1-2H3,(H,30,33)/t19-,26+,27+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501766

(CHEMBL4070896)Show SMILES CC(C)(N)[C@H](NC1COCc2cc(ccc12)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C27H34N4O4/c1-27(2,28)25(26(32)30-33)29-24-18-35-17-22-15-20(9-10-23(22)24)6-3-19-4-7-21(8-5-19)16-31-11-13-34-14-12-31/h4-5,7-10,15,24-25,29,33H,11-14,16-18,28H2,1-2H3,(H,30,32)/t24?,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483386

(CHEMBL1668439)Show SMILES C[C@@H](O)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H26N4O4/c1-15(26)19(20(27)23-29)22-21(28)25-13-11-24(12-14-25)18-9-7-17(8-10-18)16-5-3-2-4-6-16/h2-10,15,19,26,29H,11-14H2,1H3,(H,22,28)(H,23,27)/t15-,19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501133

(CHEMBL3827314)Show SMILES ONC(=O)[C@@H]1CN(C(=O)O1)c1ccc(C#CC#CC2CC2)c(F)c1 |r| Show InChI InChI=1S/C17H13FN2O4/c18-14-9-13(20-10-15(16(21)19-23)24-17(20)22)8-7-12(14)4-2-1-3-11-5-6-11/h7-9,11,15,23H,5-6,10H2,(H,19,21)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC using UDP-3-O-(R-3-hydroxymyristoyl)GlcNAc as substrate measured after 60 mins by fluorescence analysis |
ACS Med Chem Lett 7: 623-8 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00057
BindingDB Entry DOI: 10.7270/Q2DV1NWV |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
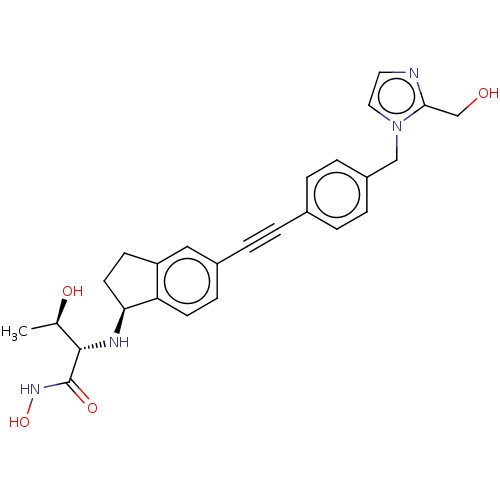
(Escherichia coli) | BDBM50501786
(CHEMBL4089353)Show SMILES [H][C@@]1(CCc2cc(ccc12)C#Cc1ccc(Cn2ccnc2CO)cc1)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C26H28N4O4/c1-17(32)25(26(33)29-34)28-23-11-9-21-14-19(8-10-22(21)23)5-2-18-3-6-20(7-4-18)15-30-13-12-27-24(30)16-31/h3-4,6-8,10,12-14,17,23,25,28,31-32,34H,9,11,15-16H2,1H3,(H,29,33)/t17-,23+,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483416

(CHEMBL1668462)Show SMILES CC(C)(O)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C22H28N4O4/c1-22(2,29)19(20(27)24-30)23-21(28)26-14-12-25(13-15-26)18-10-8-17(9-11-18)16-6-4-3-5-7-16/h3-11,19,29-30H,12-15H2,1-2H3,(H,23,28)(H,24,27)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483393

(CHEMBL1668453)Show SMILES C[C@@H](O)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)C#Cc1ccc(F)cc1)C(=O)NO |r| Show InChI InChI=1S/C23H25FN4O4/c1-16(29)21(22(30)26-32)25-23(31)28-14-12-27(13-15-28)20-10-6-18(7-11-20)3-2-17-4-8-19(24)9-5-17/h4-11,16,21,29,32H,12-15H2,1H3,(H,25,31)(H,26,30)/t16-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 21: 1155-61 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.111
BindingDB Entry DOI: 10.7270/Q2Q52SFZ |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50501775
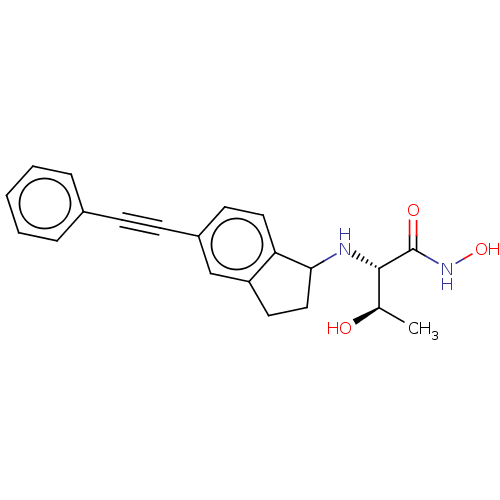
(CHEMBL4077504)Show SMILES C[C@@H](O)[C@H](NC1CCc2cc(ccc12)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O3/c1-14(24)20(21(25)23-26)22-19-12-10-17-13-16(9-11-18(17)19)8-7-15-5-3-2-4-6-15/h2-6,9,11,13-14,19-20,22,24,26H,10,12H2,1H3,(H,23,25)/t14-,19?,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Cubist Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LpxC in Escherichia coli isolate 35 ATCC 25922 |
Bioorg Med Chem Lett 27: 1670-1680 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.006
BindingDB Entry DOI: 10.7270/Q2HH6P2W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data