

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561384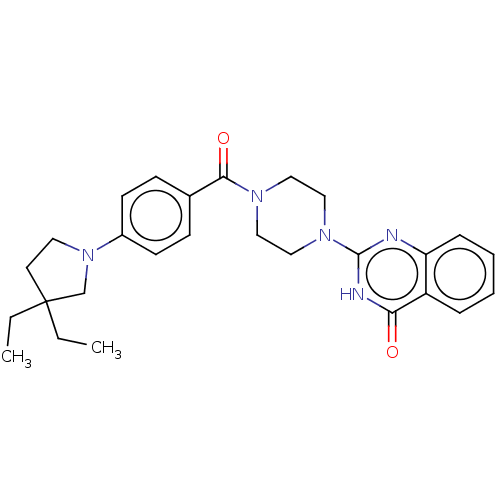 (US11390610, Example 213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561025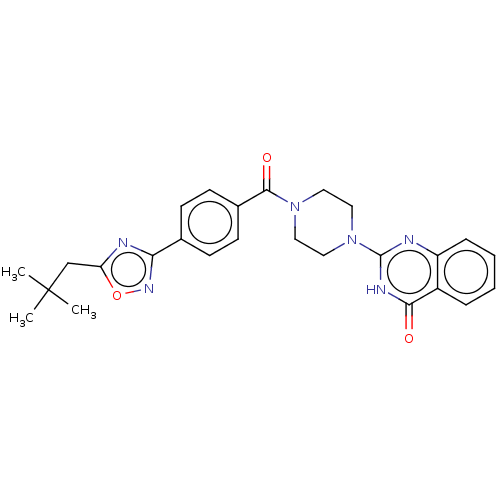 (US11390610, Example 67) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561355 (US11390610, Example 184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561217 (US11390610, Example 116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561381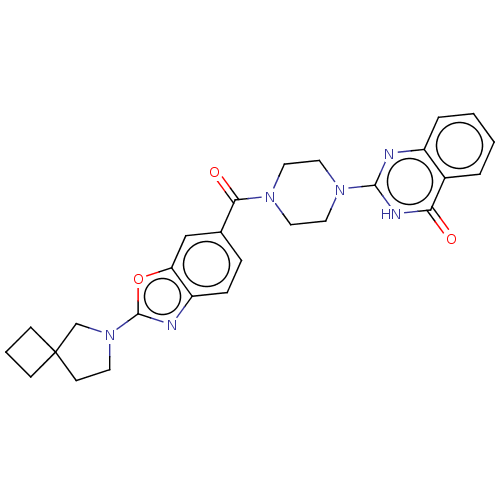 (US11390610, Example 210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561367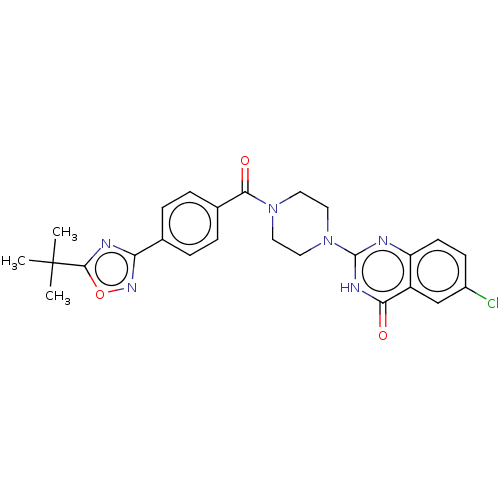 (US11390610, Example 196) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561341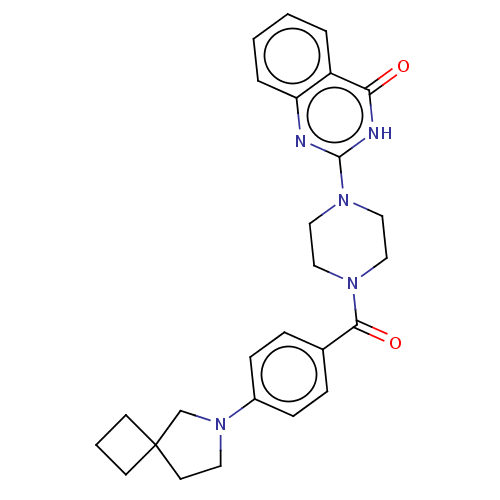 (US11390610, Example 170) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561328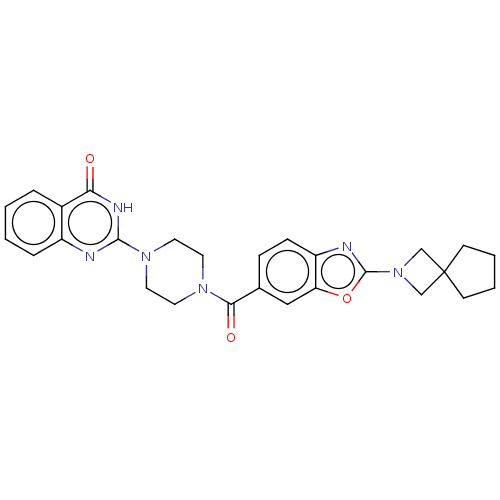 (US11390610, Example 157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561382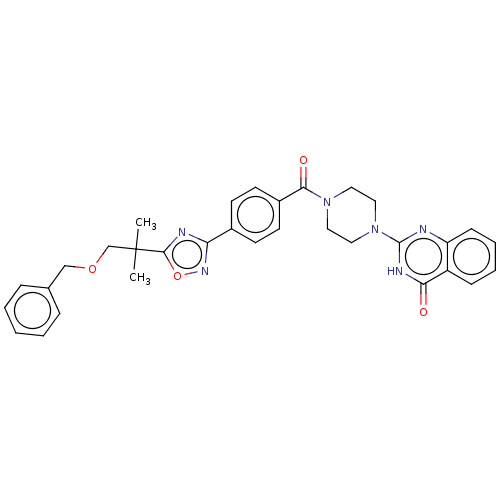 (US11390610, Example 211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561368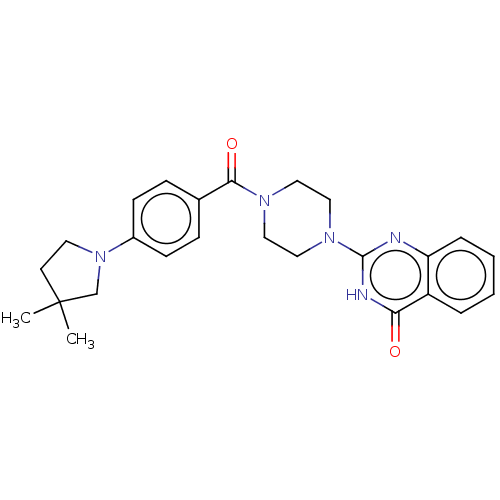 (US11390610, Example 197) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561363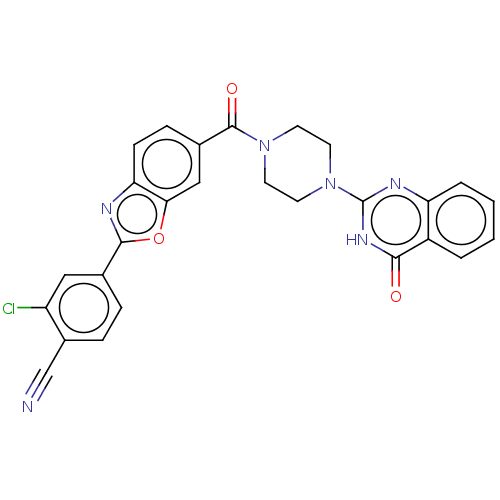 (US11390610, Example 192) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561375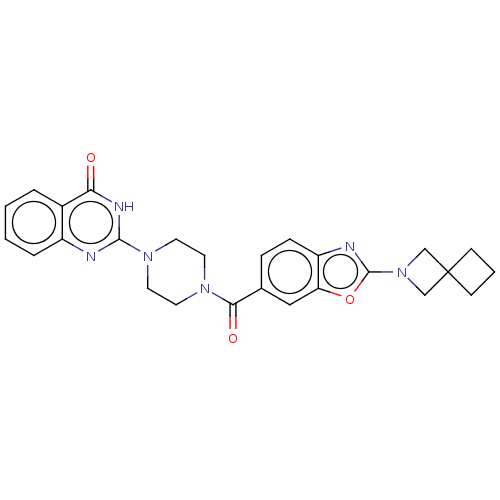 (US11390610, Example 204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561038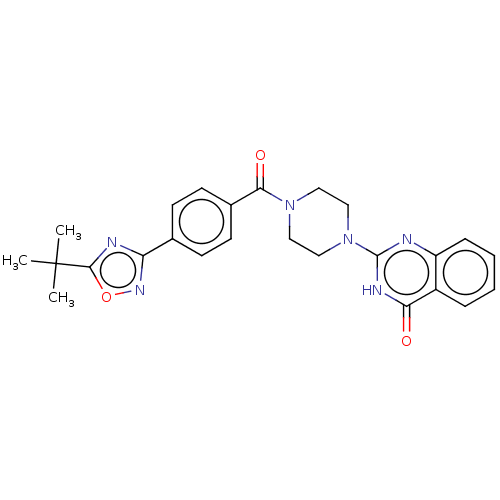 (US11390610, Example 75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561019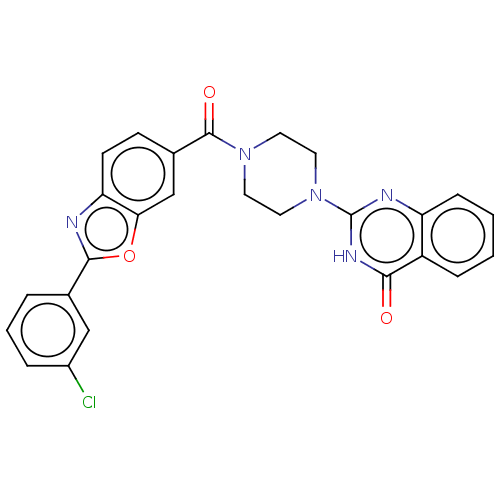 (US11390610, Example 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561299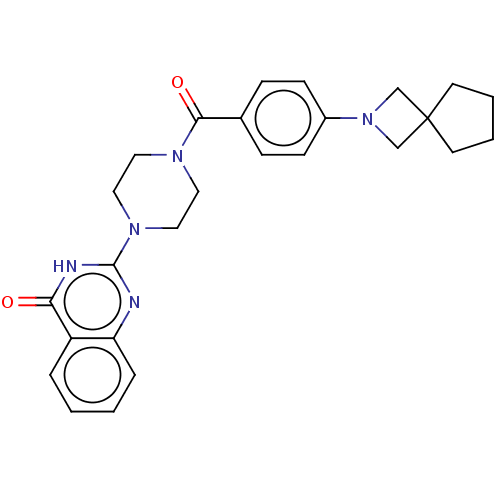 (US11390610, Example 128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561046 (US11390610, Example 83) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM560942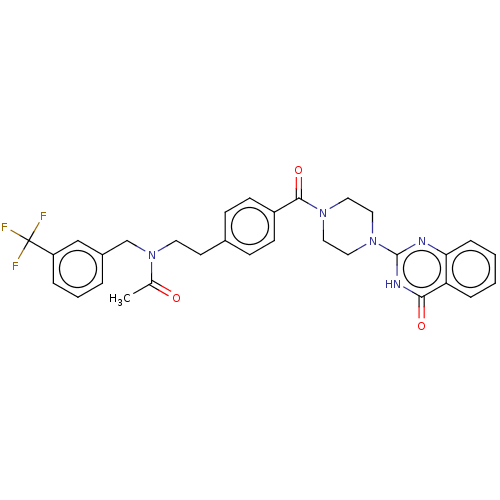 (US11390610, Example 1 | US11390610, Example 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561034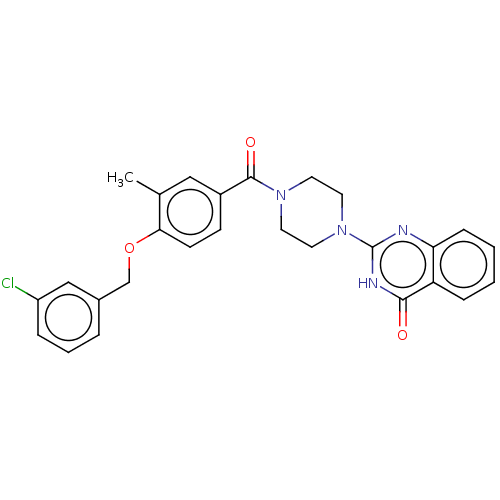 (US11390610, Example 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561068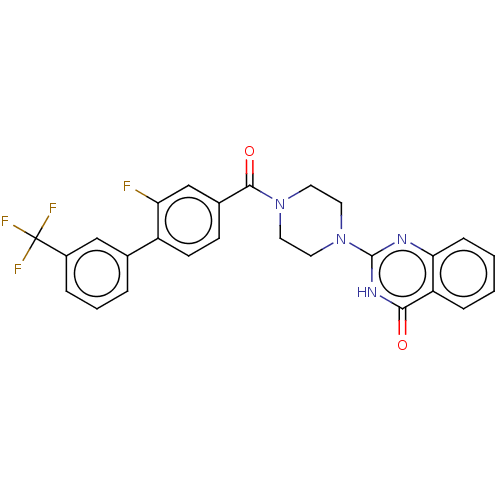 (US11390610, Example 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561373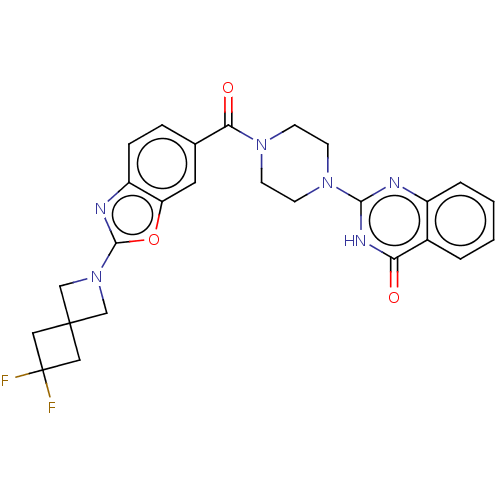 (US11390610, Example 202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561362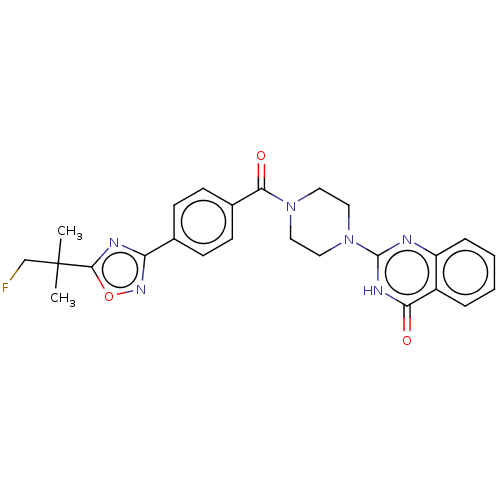 (US11390610, Example 191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561020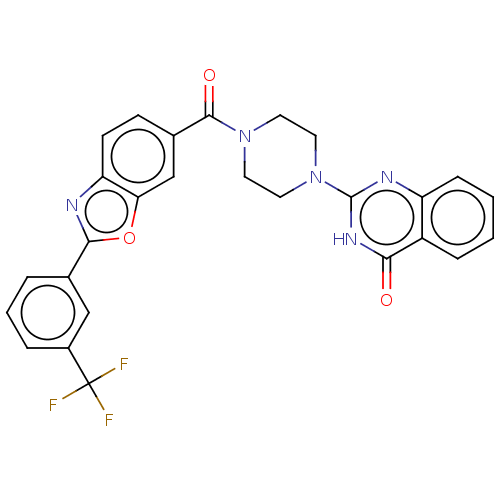 (US11390610, Example 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561364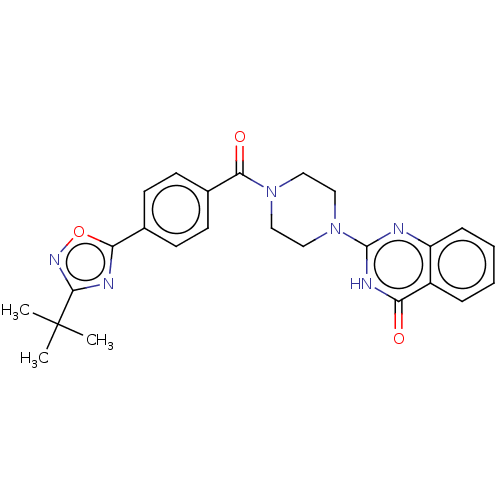 (US11390610, Example 193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561099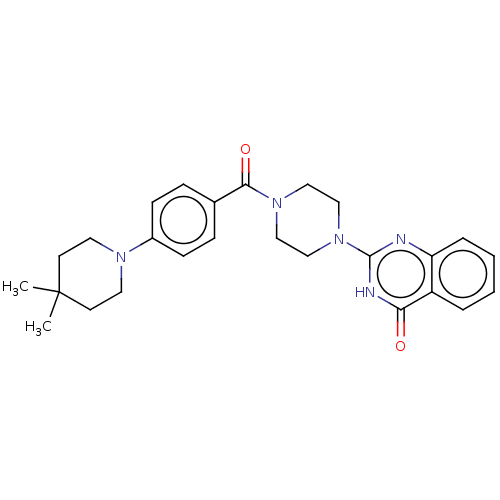 (US11390610, Example 112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561024 (US11390610, Example 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561327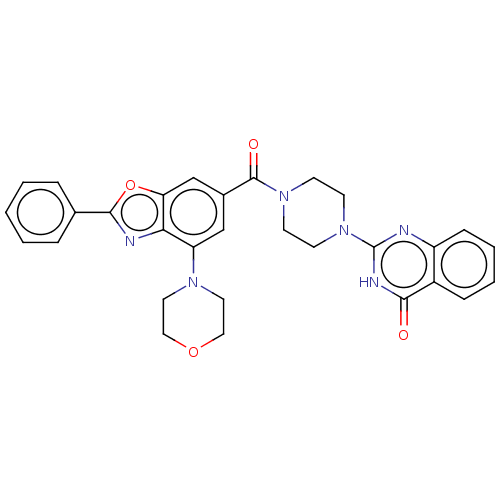 (US11390610, Example 156) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561033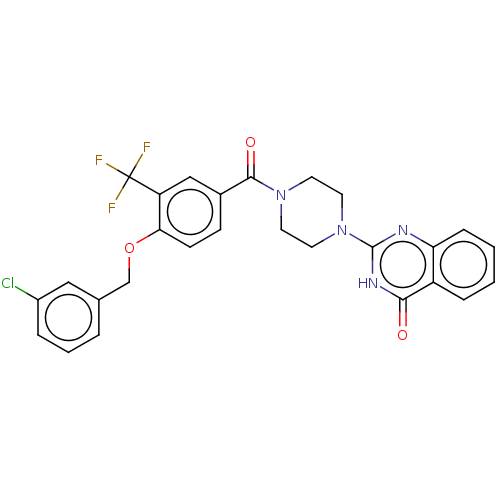 (US11390610, Example 72) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561039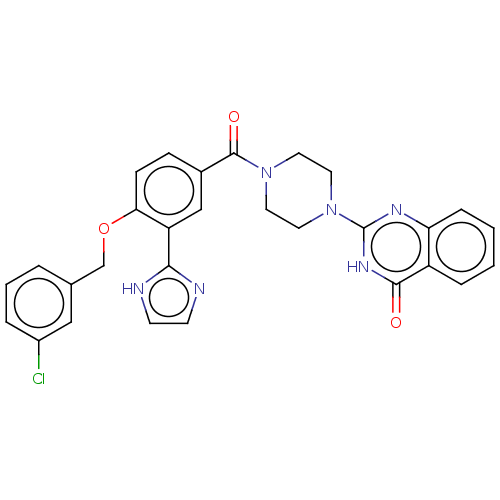 (US11390610, Example 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561366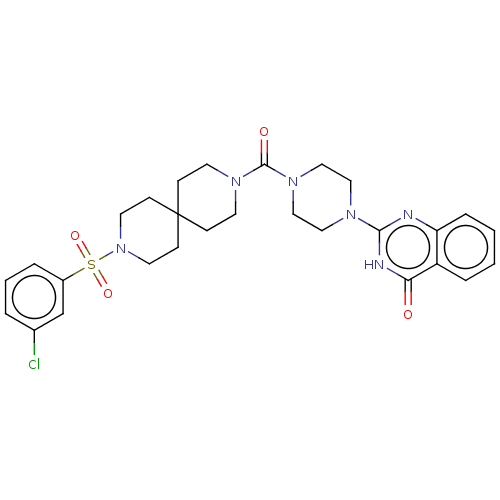 (2-[4-[3-(3-Chlorophenyl)sulfonyl-3,9-diazaspiro[5....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM560969 (US11390610, Example 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561302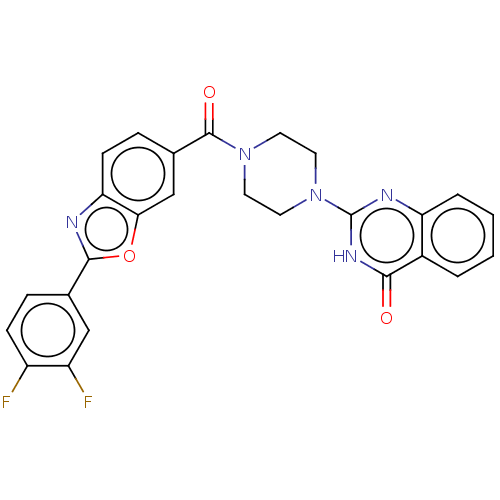 (US11390610, Example 131) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM560956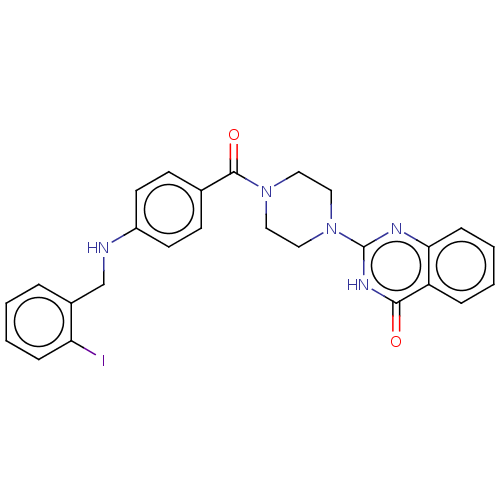 (US11390610, Example 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561329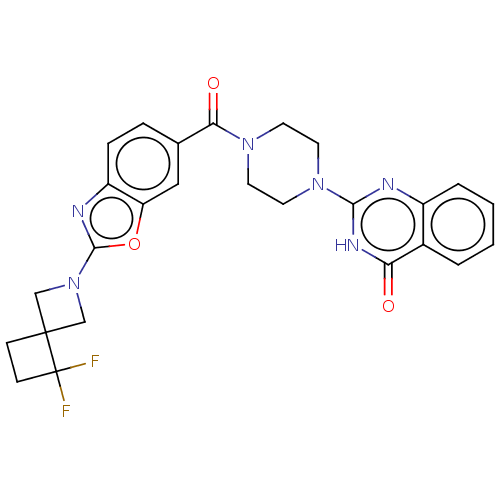 (US11390610, Example 158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561088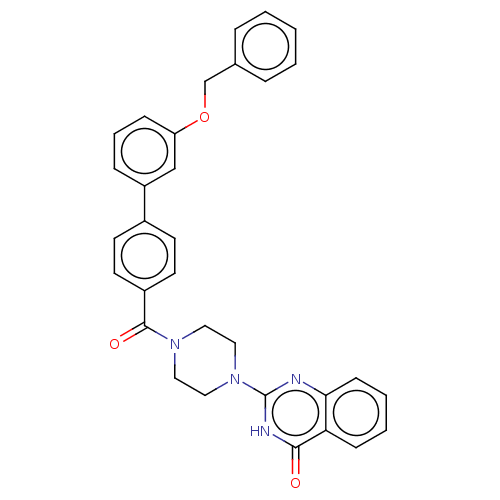 (US11390610, Example 104) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561090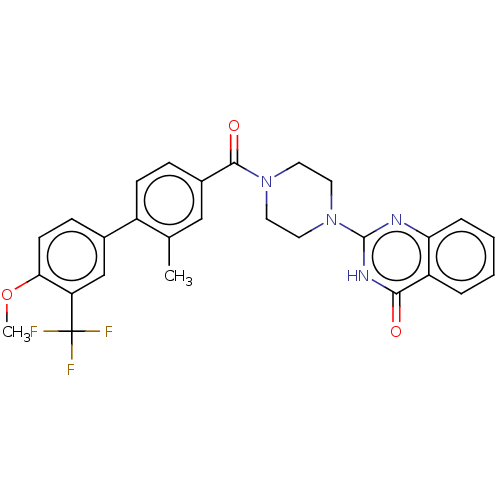 (US11390610, Example 106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561321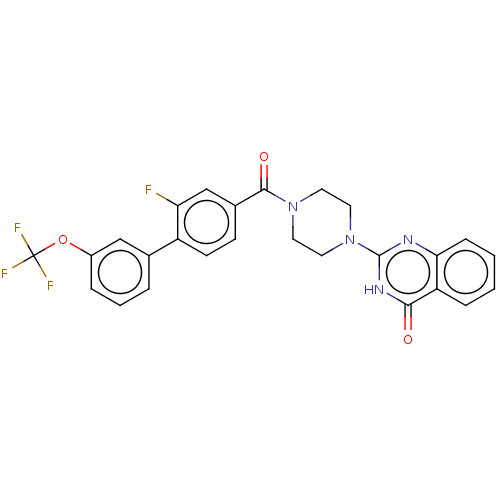 (US11390610, Example 150) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561218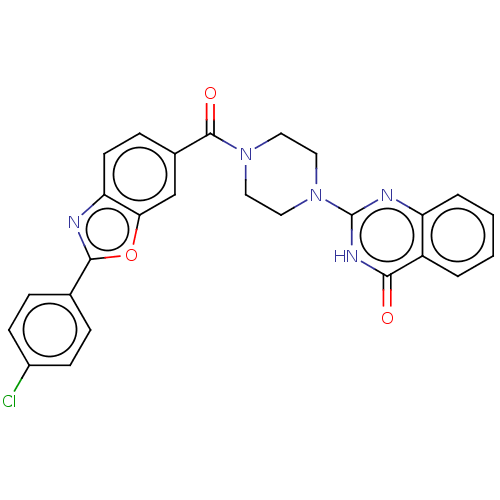 (US11390610, Example 117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561029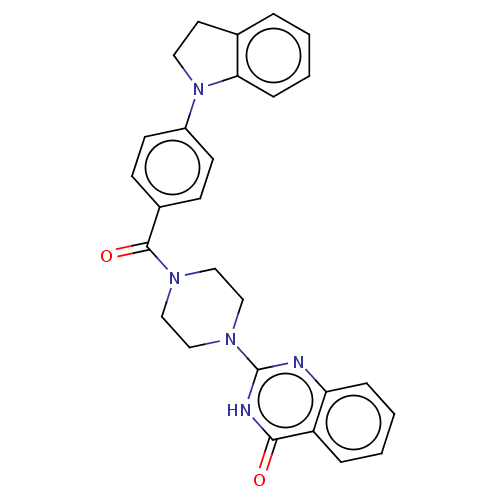 (US11390610, Example 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561011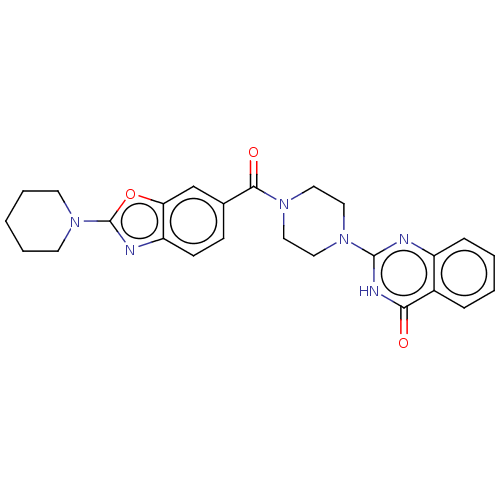 (US11390610, Example 54) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561345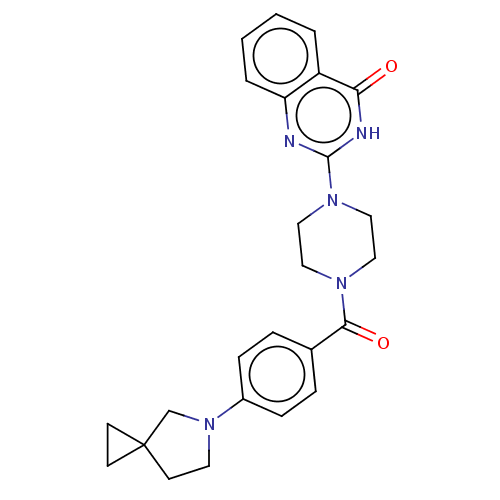 (US11390610, Example 174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561229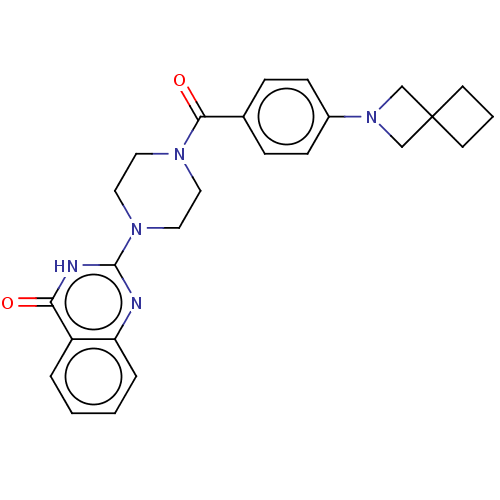 (US11390610, Example 119) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561346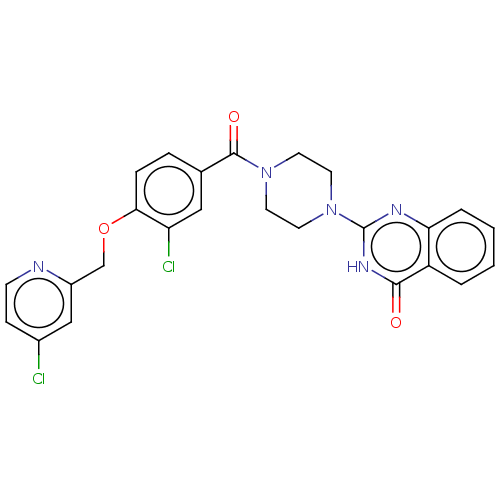 (2-[4-[3-Chloro-4-[(4-chloropyridin-2-yl)methoxy]be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM560942 (US11390610, Example 1 | US11390610, Example 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM560954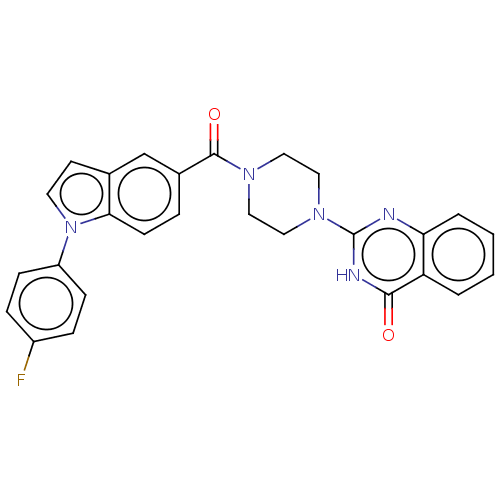 (US11390610, Example 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561306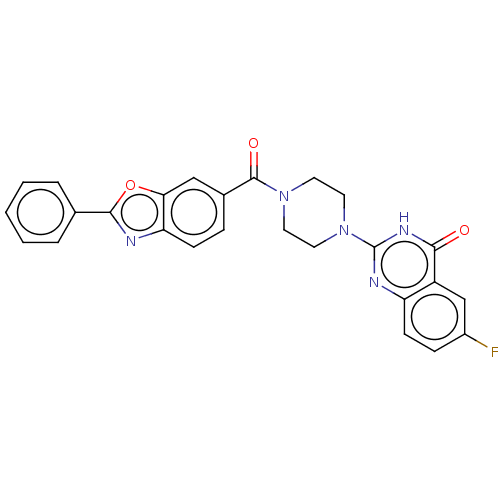 (US11390610, Example 135) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM560943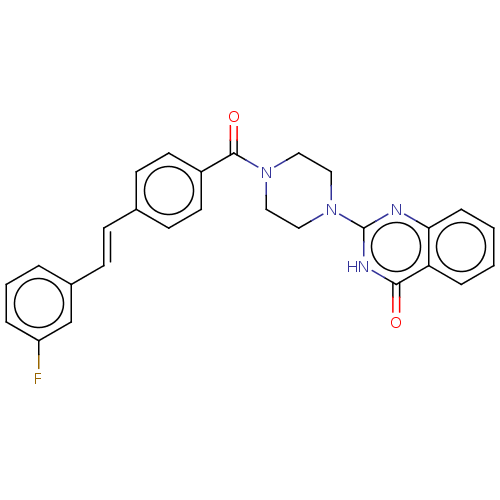 (US11390610, Example 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561298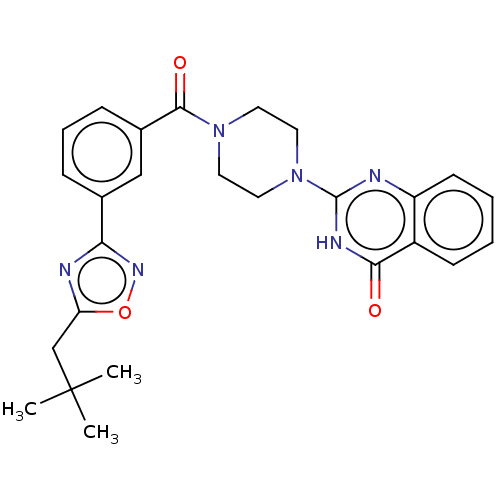 (US11390610, Example 127) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561005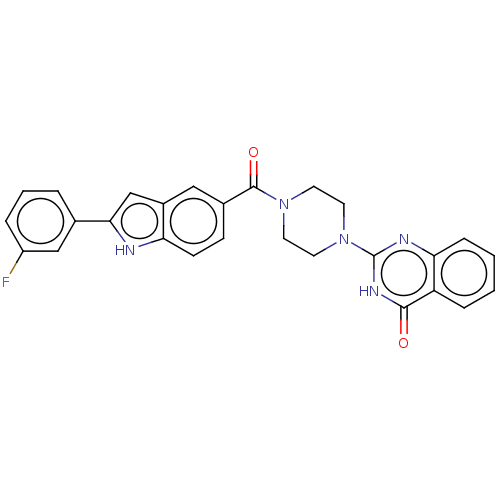 (US11390610, Example 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561340 (US11390610, Example 169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM561356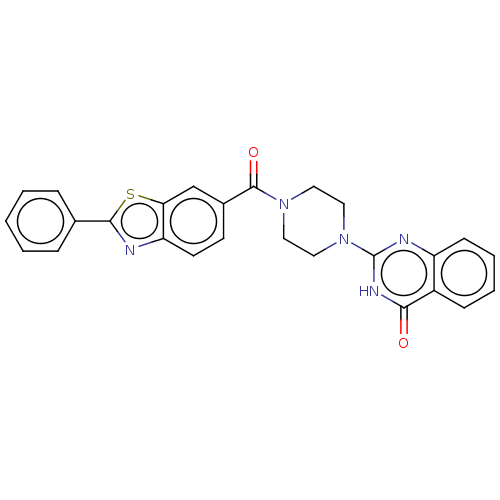 (US11390610, Example 185) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Compounds of the present invention are MAGL inhibitors. Thus, in one aspect, the present invention provides the use of compounds of Formula (I) or a ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W0994M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 216 total ) | Next | Last >> |