

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580120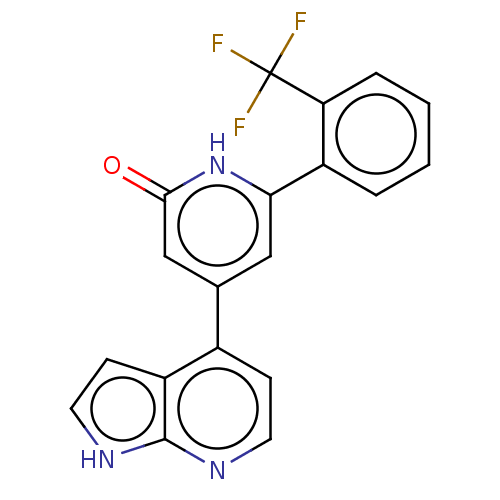 (4-(1H-pyrrolo[2,3-b]pyridin-4-yl)-6-[2-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580171 (4-(1H-pyrazolo[3,4-b]pyridin-4-yl)-6-[2-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580186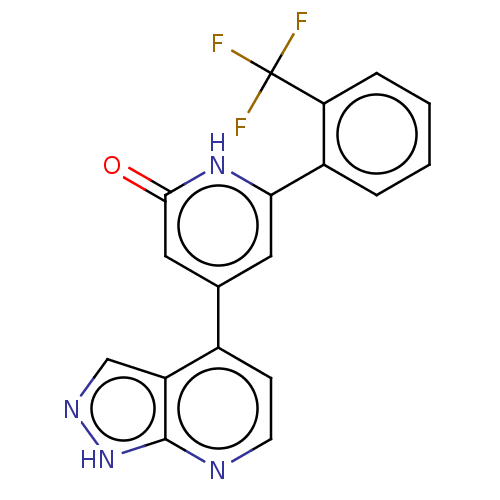 (4-(1H-pyrazolo[3,4-b]pyridin-4-yl)-6-[2-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580185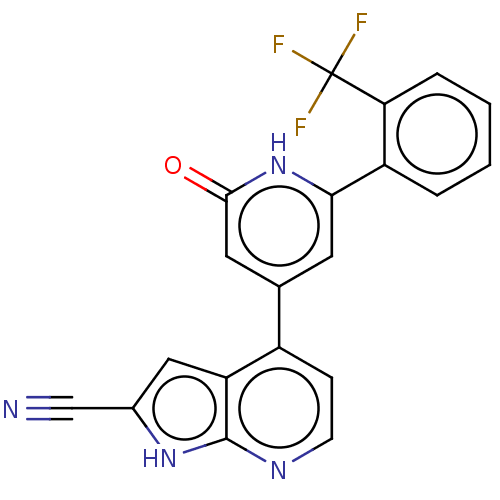 (4-[2-Oxo-6-[2-(trifluoromethyl)phenyl]-1H-pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580183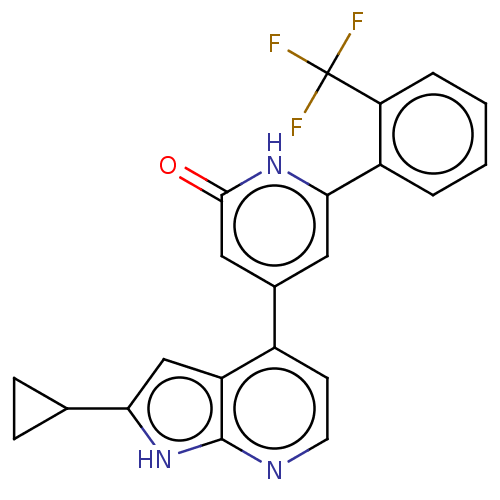 (4-(2-cyclopropyl-1H-pyrrolo[2,3-b]pyridin-4-yl)-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580182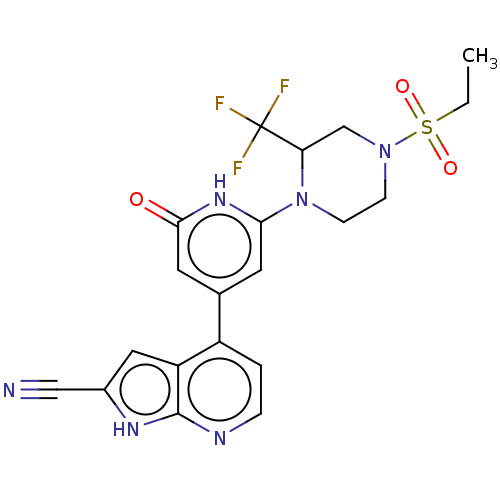 (4-[2-[4-Ethylsulfonyl-2-(trifluoromethyl)piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580179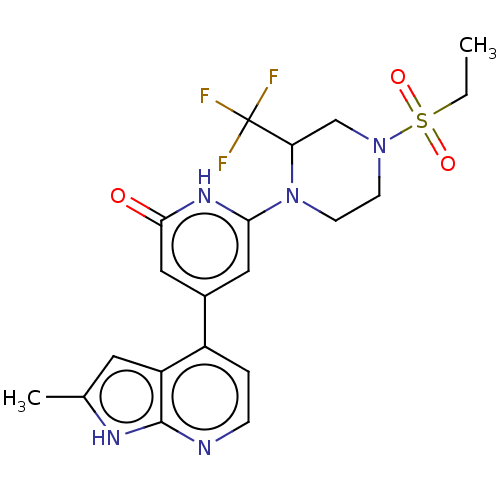 (6-[4-Ethylsulfonyl-2-(trifluoromethyl)piperazin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580178 (6-[4-[(4-fluorophenyl)methylsulfonyl]-2-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580165 (6-(2-Chlorophenyl)-4-[2-(3-pyridyl)-1H-pyrrolo[2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580166 (6-(2-Chlorophenyl)-4-(2-methyl-1H-pyrrolo[2,3-b]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580167 (4-(1H-pyrrolo[2,3-b]pyridin-4-yl)-6-[2-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580168 (4-(2-Methyl-1H-pyrrolo[2,3-b]pyridin-4-yl)-6-[2-(t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580170 (4-[2-(5-Methyl-2-thienyl)-1H-pyrrolo[2,3-b]pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580187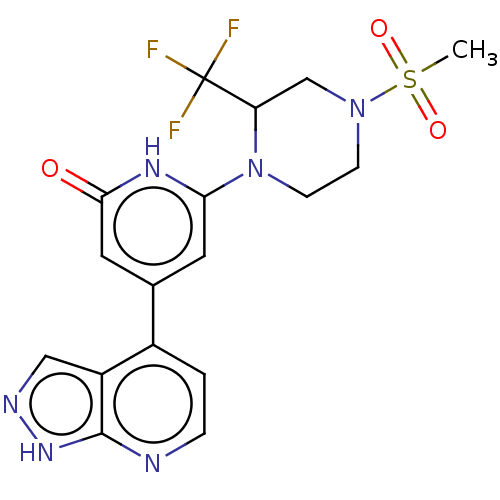 (6-[4-Methylsulfonyl-2-(trifluoromethyl)piperazin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580172 (6-[2-(Trifluoromethyl)-1-piperidyl]-4-[2-[2-(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580173 (4-(1H-pyrrolo[2,3-b]pyridin-4-yl)-6-[3-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580174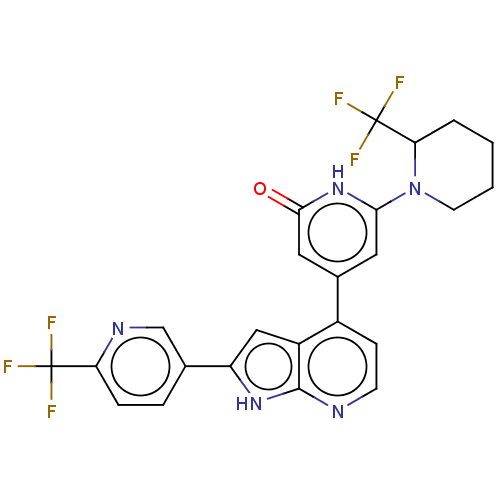 (6-[2-(Trifluoromethyl)-1-piperidyl]-4-[2-[6-(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580176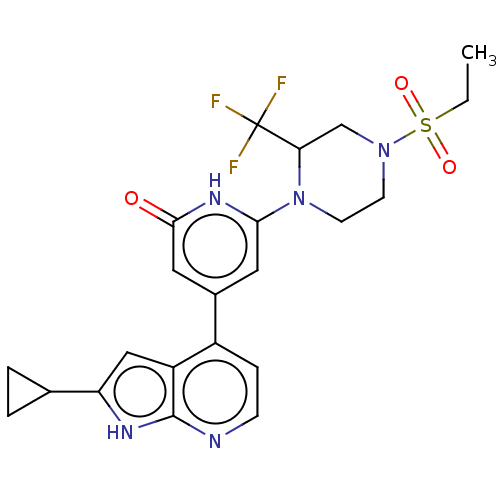 (4-(2-Cyclopropyl-1H-pyrrolo[2,3-b]pyridin-4-yl)-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580177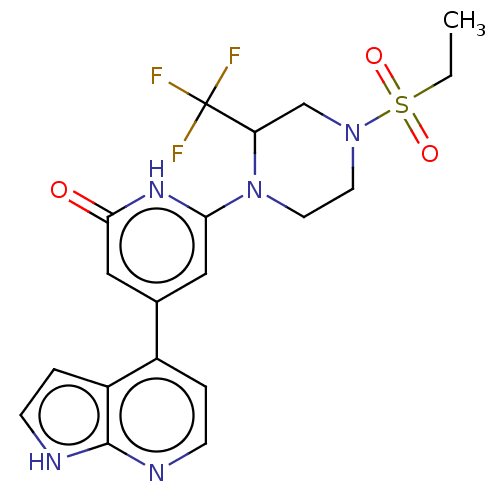 (6-[4-Ethylsulfonyl-2-(trifluoromethyl)piperazin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580175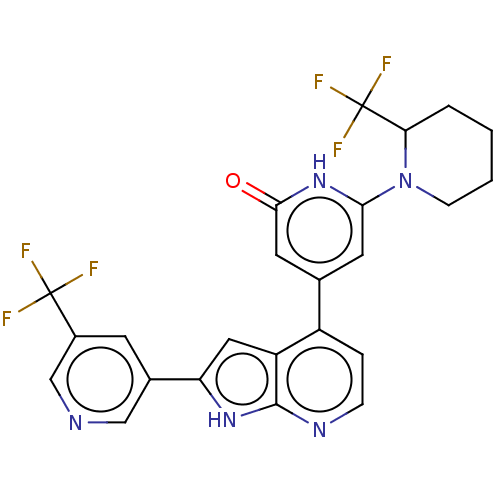 (6-[2-(Trifluoromethyl)-1-piperidyl]-4-[2-[5-(trifl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580164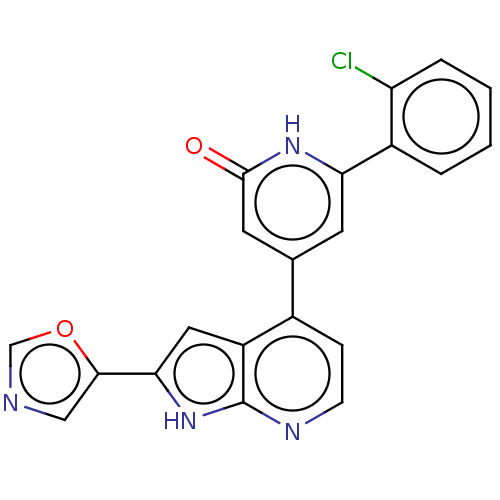 (6-(2-Chlorophenyl)-4-(2-oxazol-5-yl-1H-pyrrolo[2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580121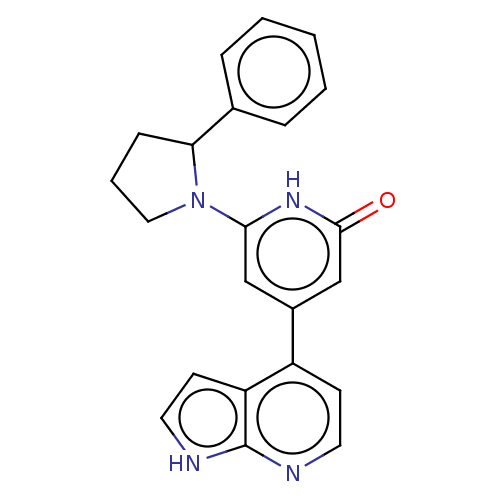 (6-(2-phenylpyrrolidin-1-yl)-4-(1H-pyrrolo[2,3-b]py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580133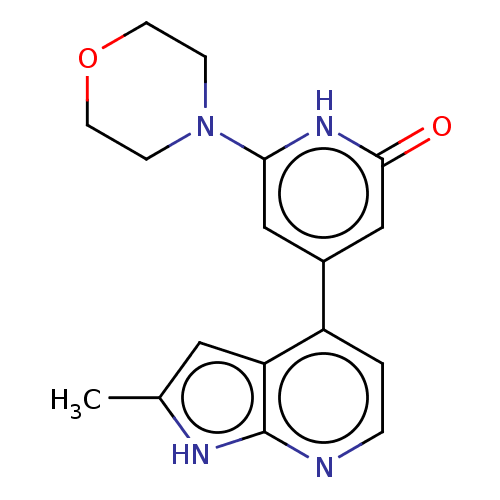 (4-(2-Methyl-1H-pyrrolo[2,3-b]pyridin-4-yl)-6-morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580122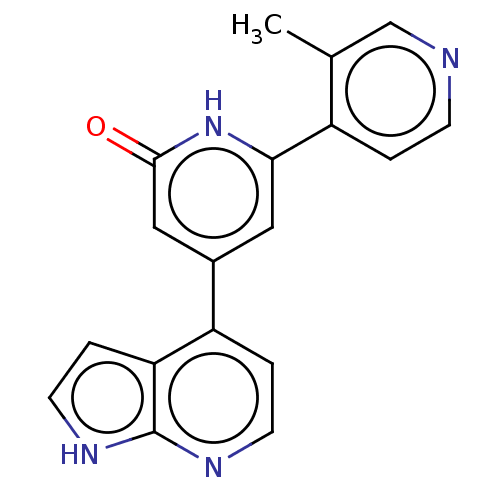 (6-(3-Methyl-4-pyridyl)-4-(1H-pyrrolo[2,3-b]pyridin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580163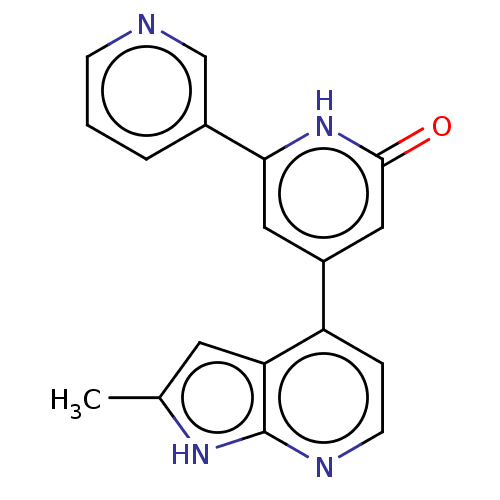 (4-(2-Methyl-1H-pyrrolo[2,3-b]pyridin-4-yl)-6-(3-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM580169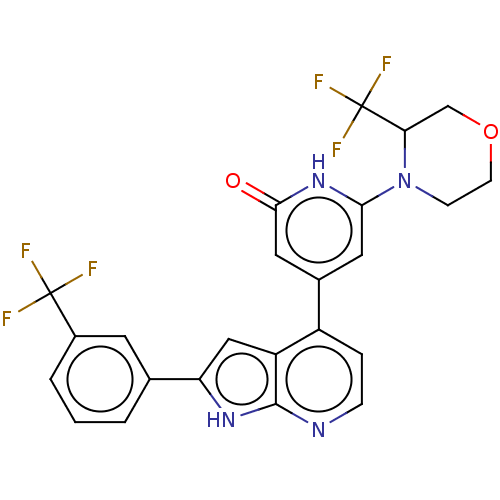 (6-[3-(Trifluoromethyl)morpholin-4-yl]-4-[2-[3-(tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q24J0JZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||