

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50446250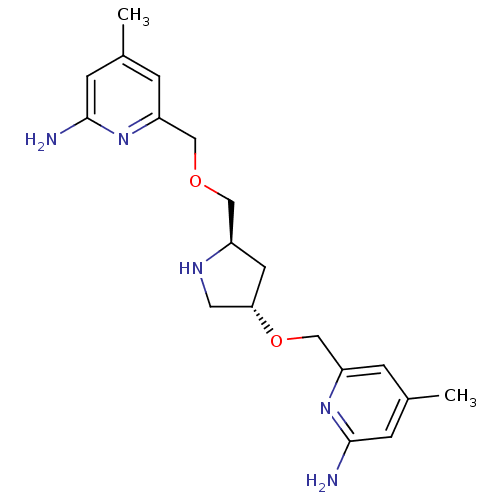 (CHEMBL3109188 | US9732037, Compound 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50446252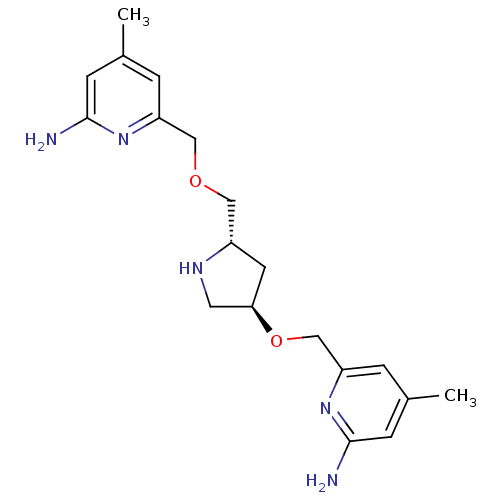 (CHEMBL3109186 | US9732037, Compound 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50446249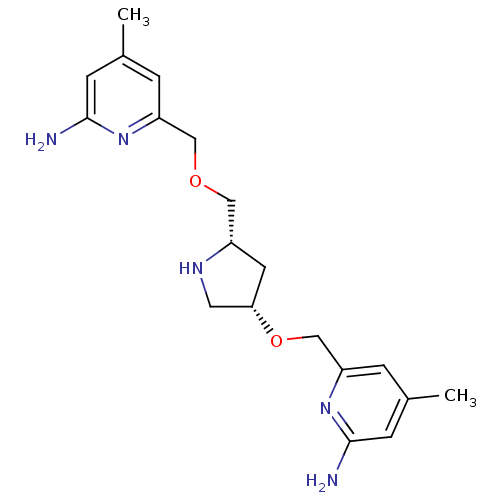 (CHEMBL3109189 | US9732037, Compound 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50441011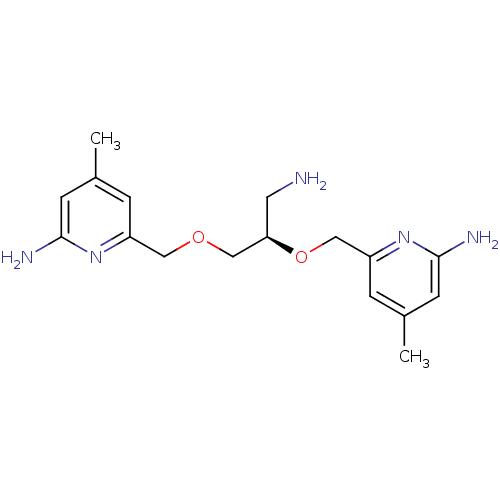 (CHEMBL2430144 | US9732037, Compound 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50446251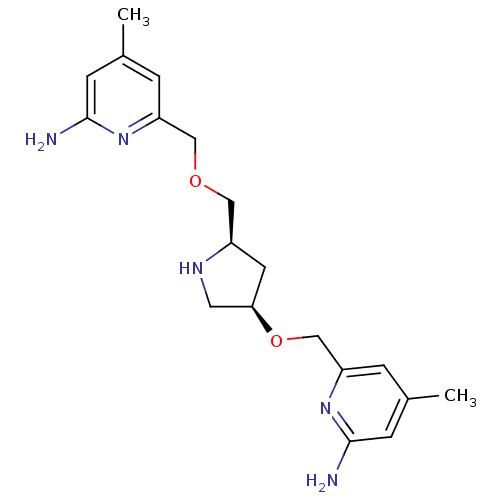 (CHEMBL3109187 | US9732037, Compound 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50441008 (CHEMBL2430150 | US9732037, Compound 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50438641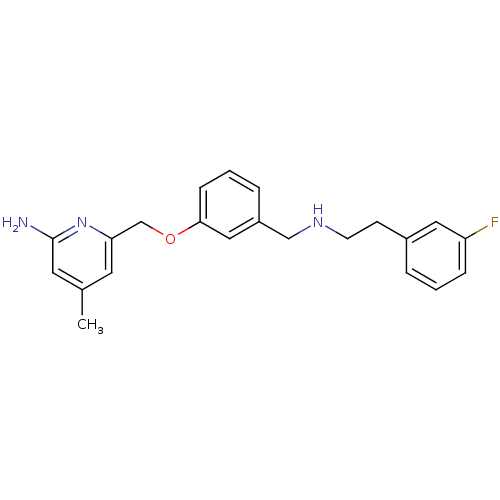 (CHEMBL2414435 | US9732037, Compound 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50441007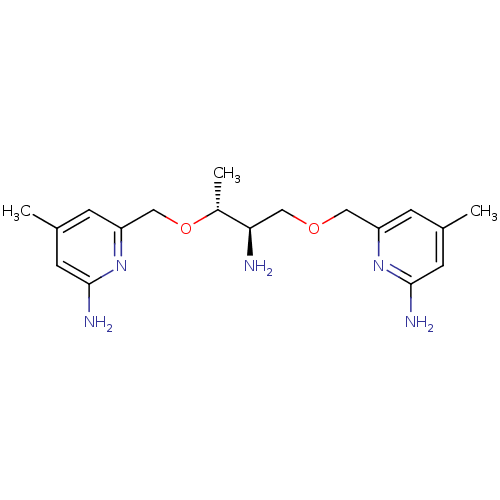 (CHEMBL2430149 | US9732037, Compound 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50438644 (CHEMBL2414429 | US9732037, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50446248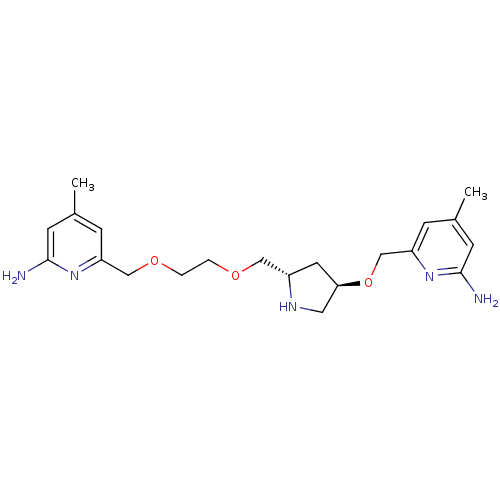 (CHEMBL3109190 | US9732037, Compound 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50438642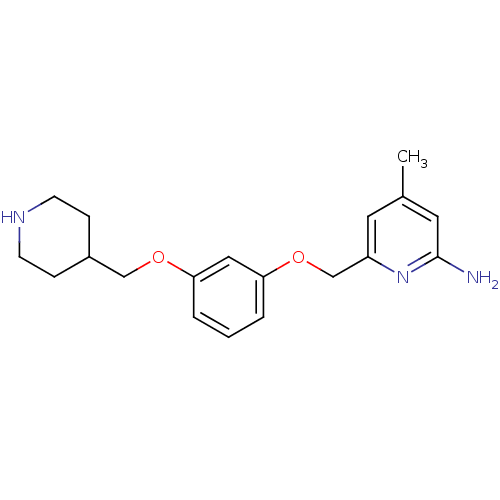 (CHEMBL2414432 | US9732037, Compound 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50438639 (CHEMBL2414437 | US9732037, Compound 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50446246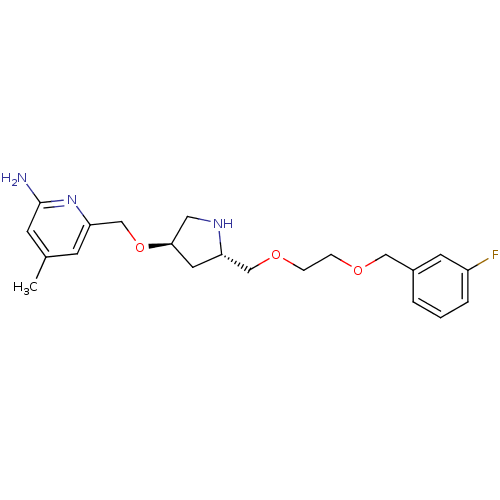 (CHEMBL3109192 | US9732037, Compound 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50446247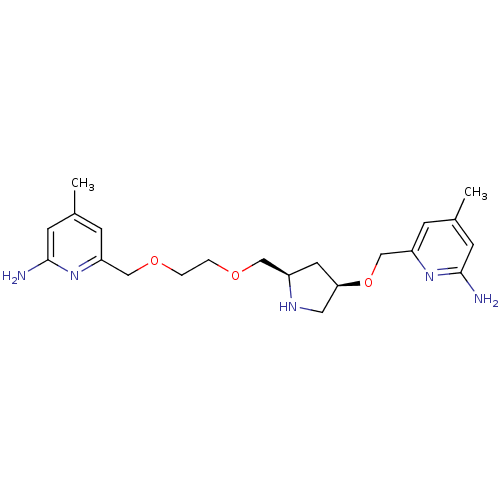 (CHEMBL3109191 | US9732037, Compound 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50441015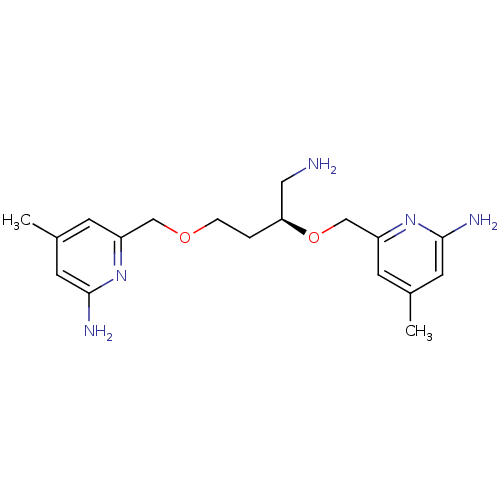 (CHEMBL2430148 | US9732037, Compound 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50446245 (CHEMBL3109193 | US9732037, Compound 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50441010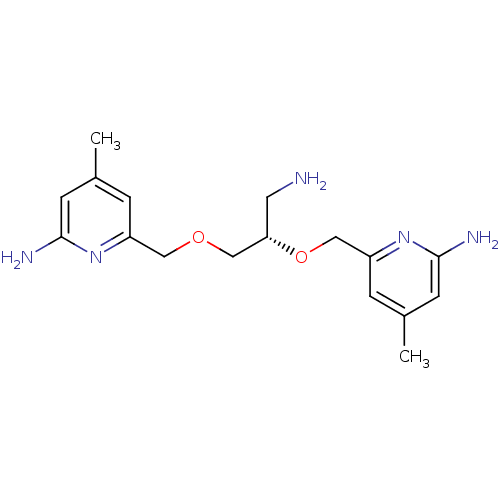 (CHEMBL2430143 | US9732037, Compound 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 382 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50441009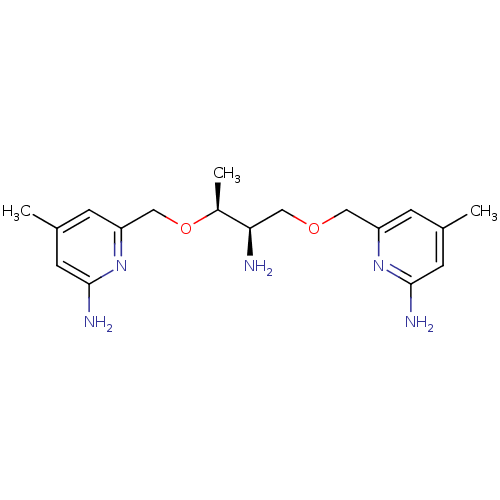 (CHEMBL2430151 | US9732037, Compound 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 384 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50438643 (CHEMBL2414430 | US9732037, Compound 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 616 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50441014 (CHEMBL2430147 | US9732037, Compound 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 881 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50441013 (CHEMBL2430146 | US9732037, Compound 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50441007 (CHEMBL2430149 | US9732037, Compound 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50446249 (CHEMBL3109189 | US9732037, Compound 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50446252 (CHEMBL3109186 | US9732037, Compound 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50441008 (CHEMBL2430150 | US9732037, Compound 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50446252 (CHEMBL3109186 | US9732037, Compound 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50446248 (CHEMBL3109190 | US9732037, Compound 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50446249 (CHEMBL3109189 | US9732037, Compound 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50438639 (CHEMBL2414437 | US9732037, Compound 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | 2.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50446250 (CHEMBL3109188 | US9732037, Compound 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | 2.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50441007 (CHEMBL2430149 | US9732037, Compound 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50441008 (CHEMBL2430150 | US9732037, Compound 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 3.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50446251 (CHEMBL3109187 | US9732037, Compound 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50446251 (CHEMBL3109187 | US9732037, Compound 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 5.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50446246 (CHEMBL3109192 | US9732037, Compound 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 5.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50446248 (CHEMBL3109190 | US9732037, Compound 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50446250 (CHEMBL3109188 | US9732037, Compound 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB US Patent | 6.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50438639 (CHEMBL2414437 | US9732037, Compound 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 7.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50441011 (CHEMBL2430144 | US9732037, Compound 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50446246 (CHEMBL3109192 | US9732037, Compound 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 7.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50438644 (CHEMBL2414429 | US9732037, Compound 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | 8.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50446245 (CHEMBL3109193 | US9732037, Compound 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 8.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50446247 (CHEMBL3109191 | US9732037, Compound 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50438641 (CHEMBL2414435 | US9732037, Compound 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | 9.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50441015 (CHEMBL2430148 | US9732037, Compound 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50441009 (CHEMBL2430151 | US9732037, Compound 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50446245 (CHEMBL3109193 | US9732037, Compound 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50446247 (CHEMBL3109191 | US9732037, Compound 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50438641 (CHEMBL2414435 | US9732037, Compound 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50441011 (CHEMBL2430144 | US9732037, Compound 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University US Patent | Assay Description NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre... | US Patent US9732037 (2017) BindingDB Entry DOI: 10.7270/Q26W9D6H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |