

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369905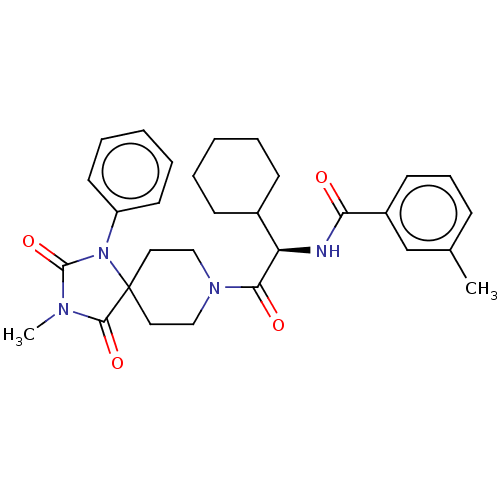 ((R)—N-(1-Cyclohexyl-2-(3-methyl-2,4-dioxo-1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369906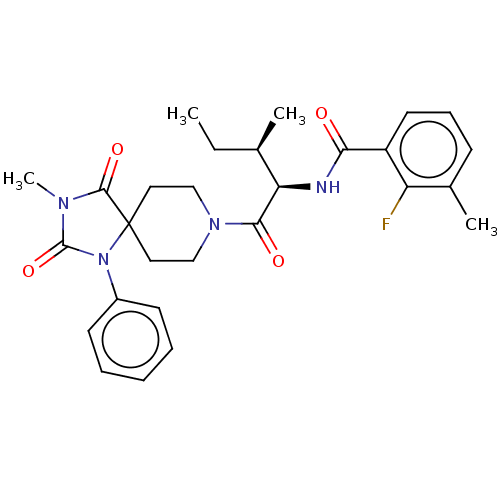 (2-Fluoro-3-methyl-N-((2R,3R)-3-methyl-1-(3-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369909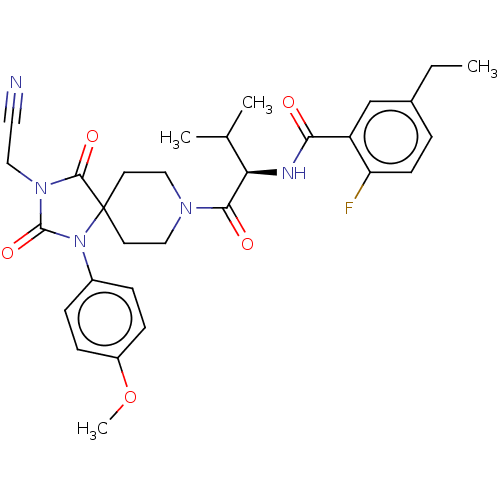 ((R)—N-(1-(3-(Cyanomethyl)-1-(4-methoxyphenyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369910 ((R)-2-Fluoro-N-(1-(1-(3-fluoro-4-methoxyphenyl)-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369911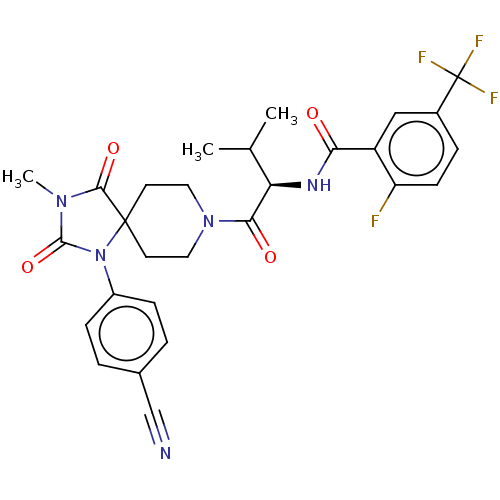 ((R)—N-(1-(1-(4-Cyanophenyl)-3-methyl-2,4-diox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369912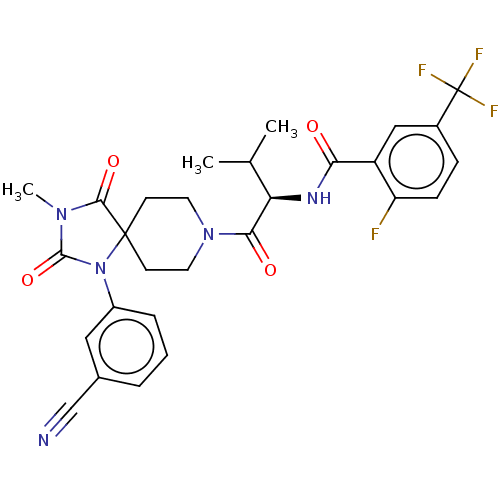 ((R)—N-(1-(1-(3-Cyanophenyl)-3-methyl-2,4-diox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369913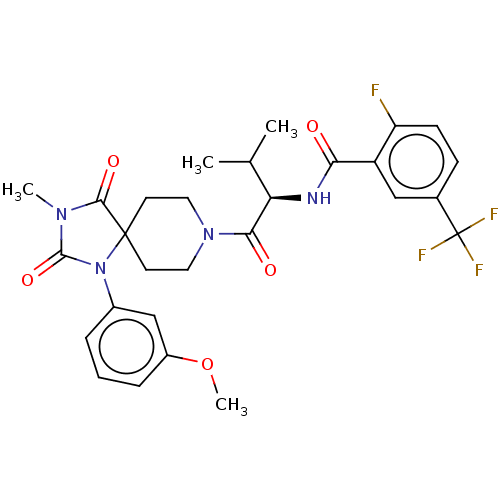 ((R)-2-Fluoro-N-(1-(1-(3-methoxyphenyl)-3-methyl-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369914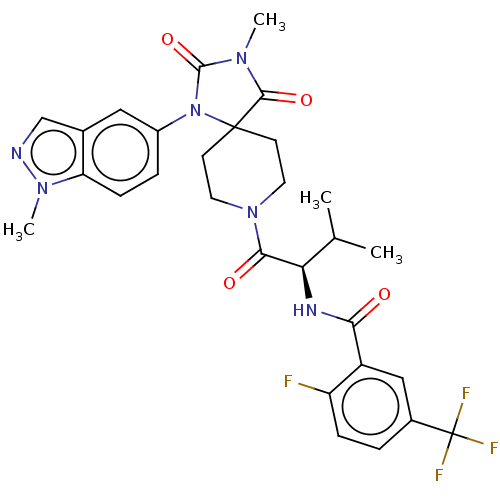 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-1-(1-methyl-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369915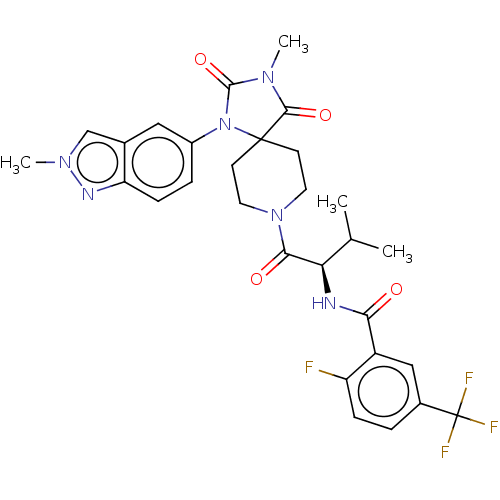 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-1-(2-methyl-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369916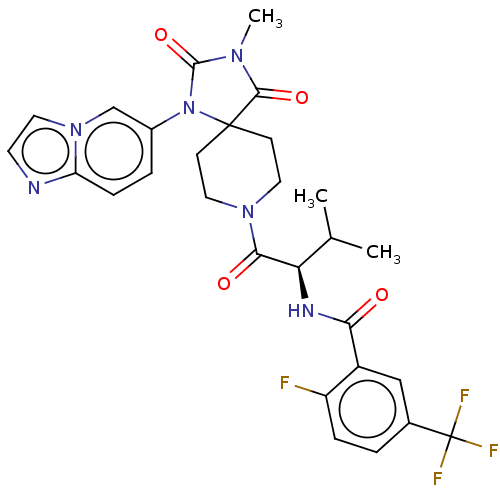 ((R)-2-Fluoro-N-(1-(1-(imidazo[1,2-a]pyridin-6-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369918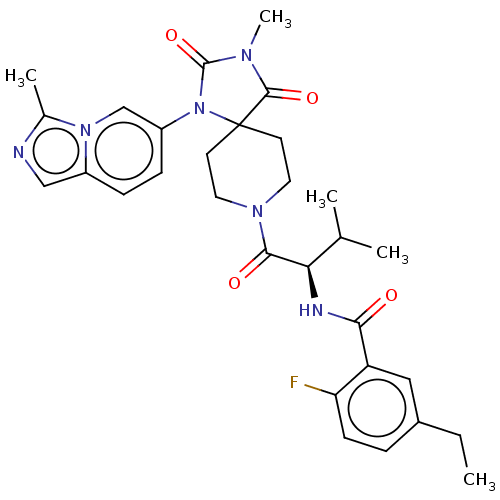 ((R)-5-Ethyl-2-fluoro-N-(3-methyl-1-(3-methyl-1-(3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369919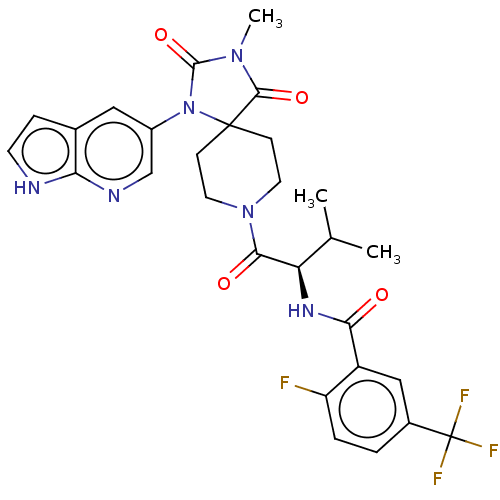 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-2,4-dioxo-1-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369920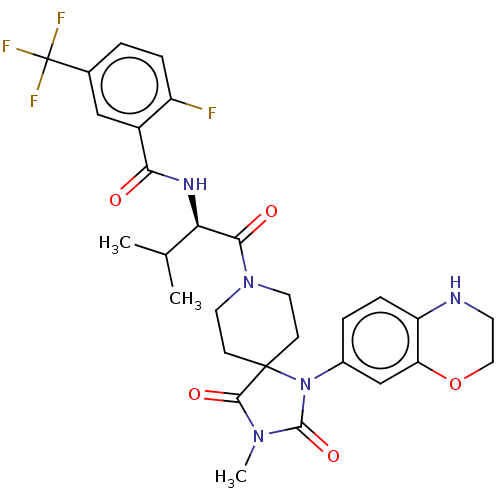 ((R)—N-(1-(1-(3,4-Dihydro-2H-benzo[b][1,4]oxaz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369921 ((R)—N-(1-Cyclopentyl-2-(3-methyl-1-(1-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369922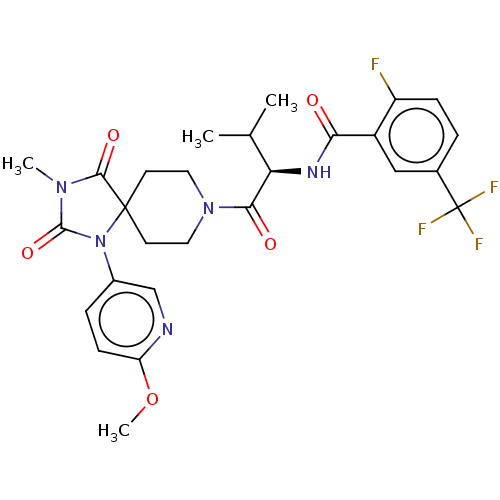 ((R)-2-Fluoro-N-(1-(1-(6-methoxypyridin-3-yl)-3-met...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369923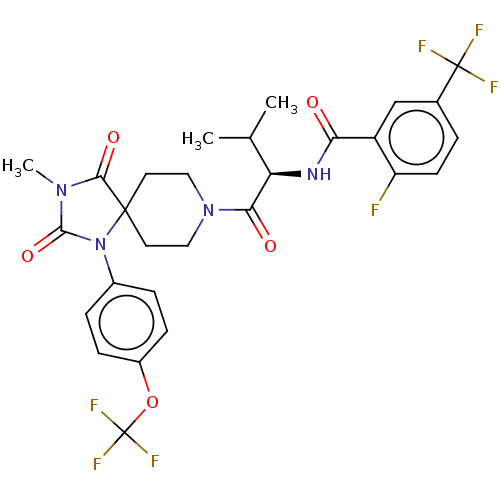 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-2,4-dioxo-1-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369924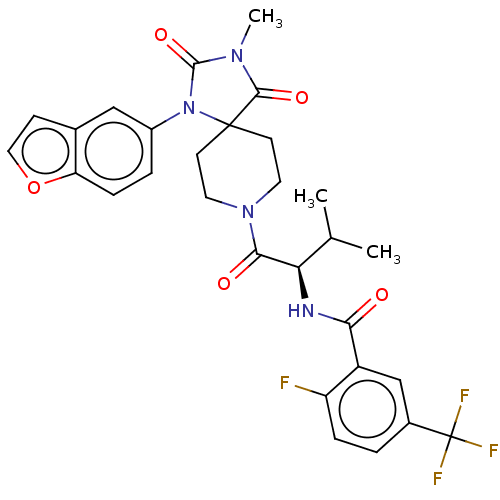 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-2,4-dioxo-1-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369925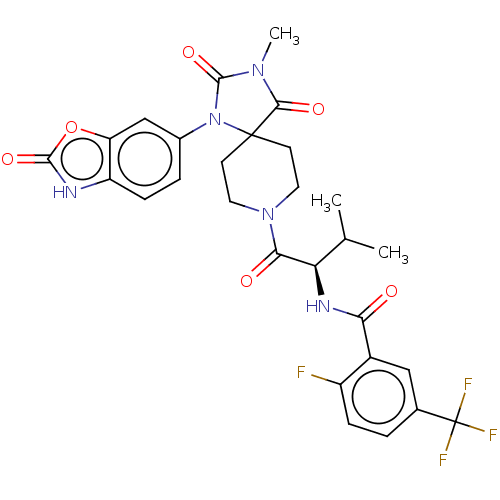 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-2,4-dioxo-1-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369926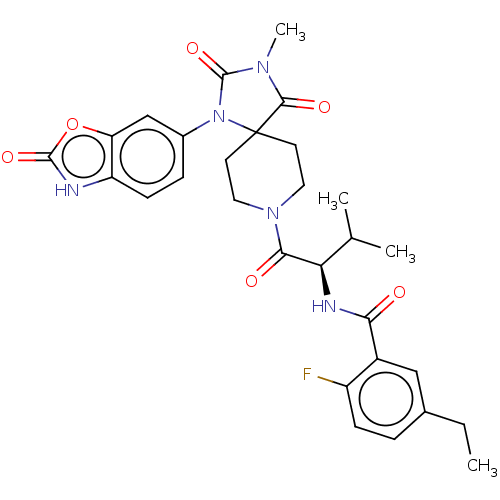 ((R)-5-Ethyl-2-fluoro-N-(3-methyl-1-(3-methyl-2,4-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369927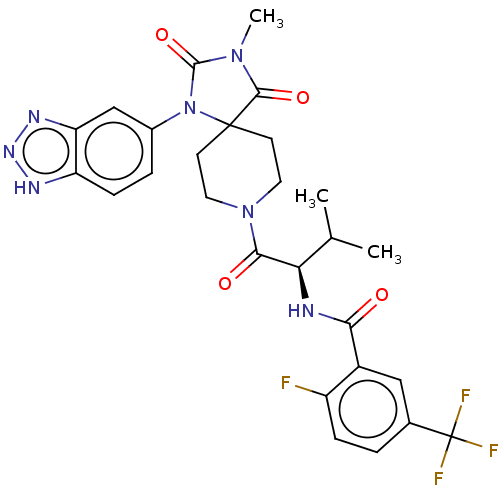 ((R)—N-(1-(1-(1H-Benzo[d][1,2,3]triazol-5-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369928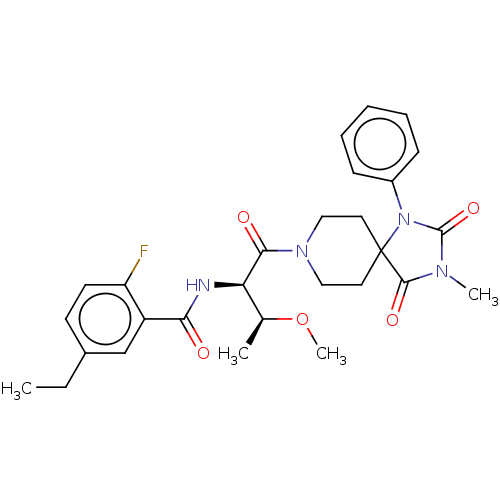 (5-Ethyl-2-fluoro-N-((2R,3S)-3-methoxy-1-(3-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369929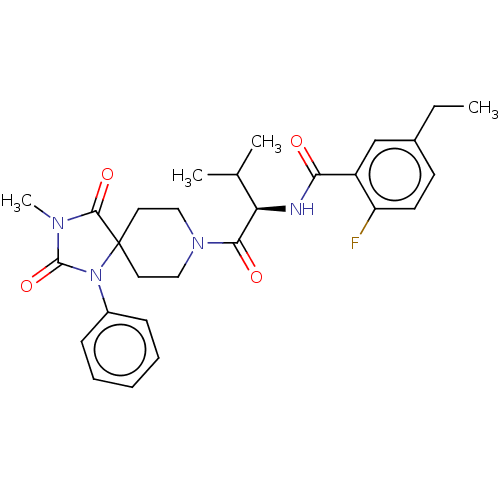 ((R)-5-Ethyl-2-fluoro-N-(3-methyl-1-(3-methyl-2,4-d...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369930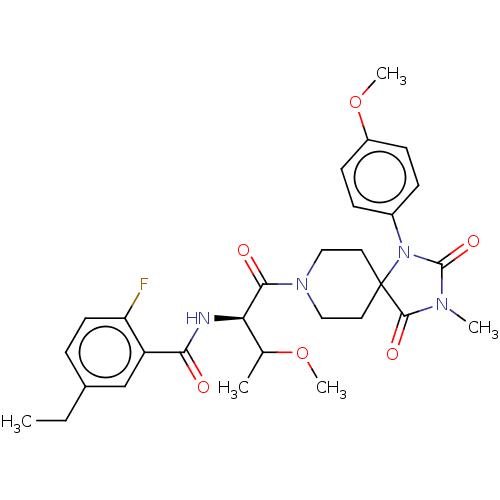 (5-Ethyl-2-fluoro-N-((2R,3R)-3-methoxy-1-(1-(4-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369931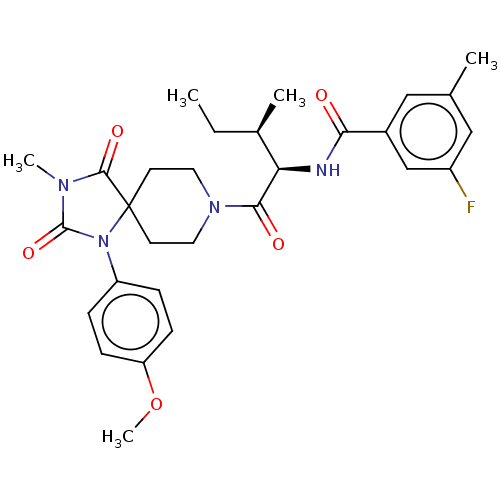 (3-Fluoro-N-((2R,3R)-1-(1-(4-methoxyphenyl)-3-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369932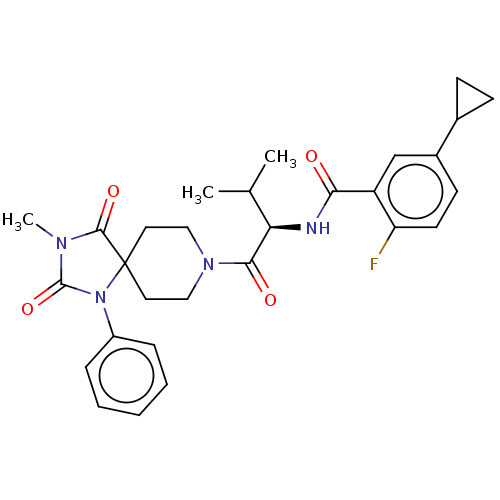 ((R)-5-Cyclopropyl-2-fluoro-N-(3-methyl-1-(3-methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369933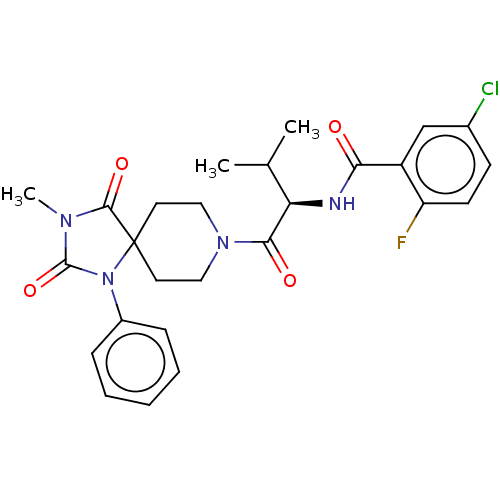 ((R)-5-Chloro-2-fluoro-N-(3-methyl-1-(3-methyl-2,4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369934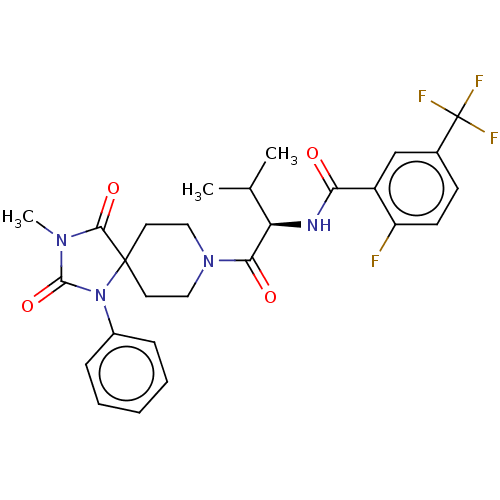 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-2,4-dioxo-1-p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369935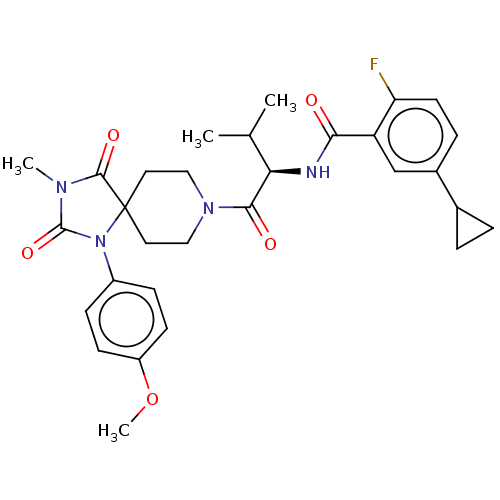 ((R)-5-Cyclopropyl-2-fluoro-N-(1-(1-(4-methoxypheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369936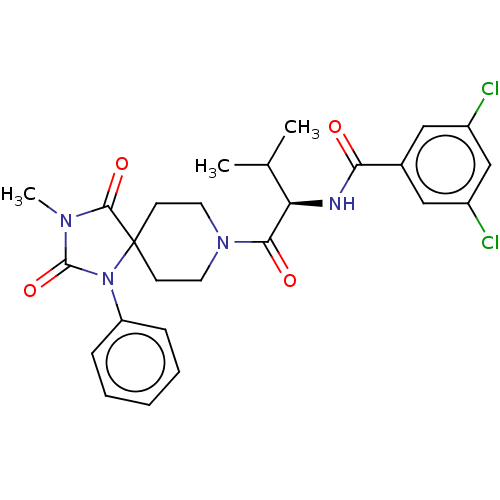 ((R)-3,5-Dichloro-N-(3-methyl-1-(3-methyl-2,4-dioxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369937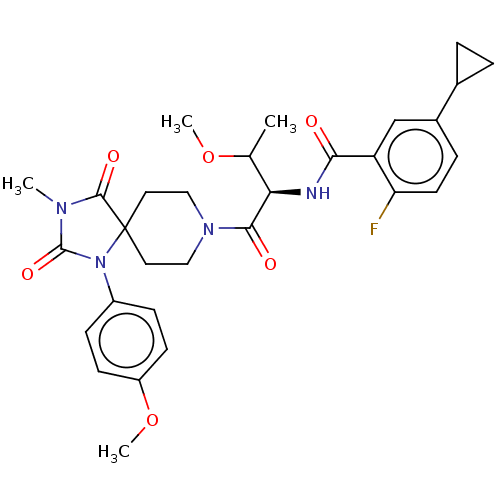 (5-Cyclopropyl-2-fluoro-N-((2R,3R)-3-methoxy-1-(1-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369938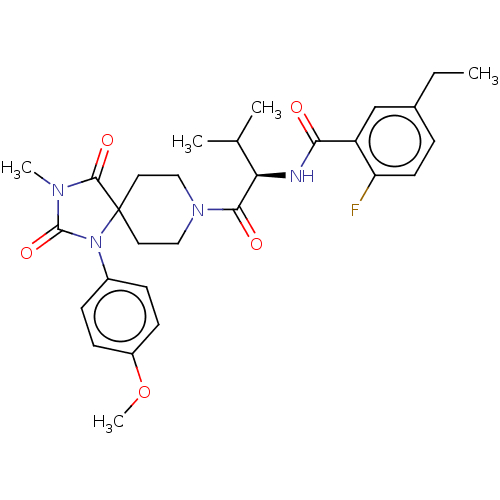 ((R)-5-Ethyl-2-fluoro-N-(1-(1-(4-methoxyphenyl)-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369939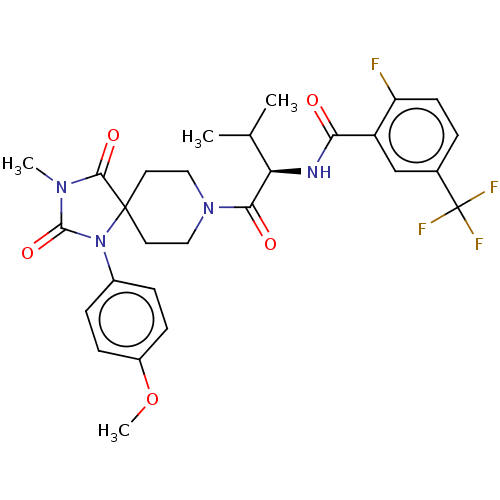 ((R)-2-Fluoro-N-(1-(1-(4-methoxyphenyl)-3-methyl-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369940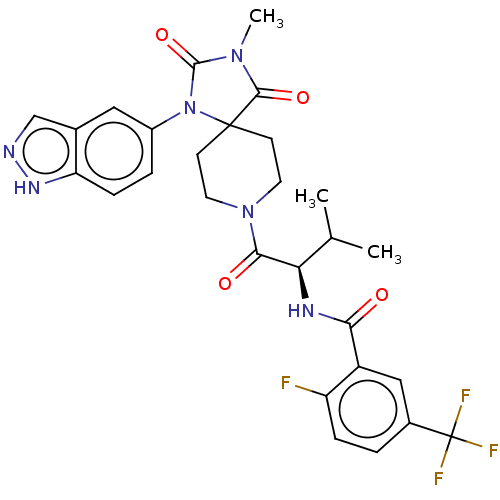 ((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369941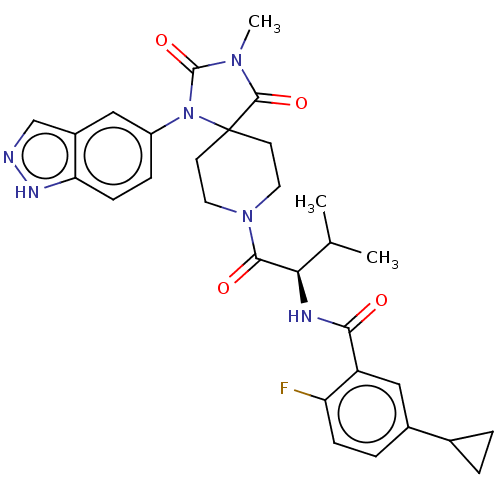 ((R)—N-(1-(1-(1H-Indazol-5-yl)-3-methyl-2,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369942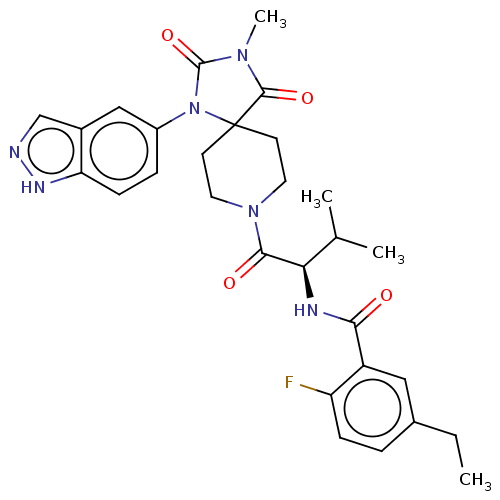 ((R)—N-(1-(1-(1H-Indazol-5-yl)-3-methyl-2,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369943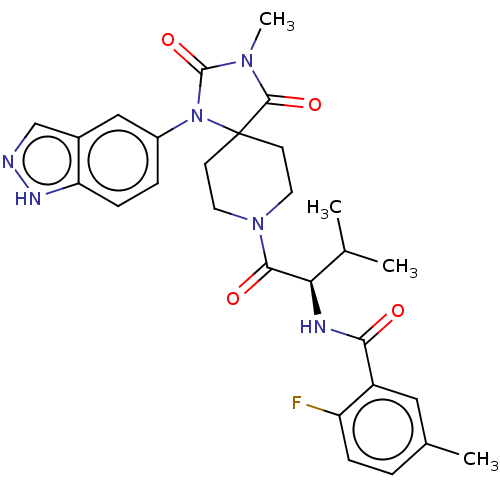 ((R)—N-(1-(1-(1H-Indazol-5-yl)-3-methyl-2,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369944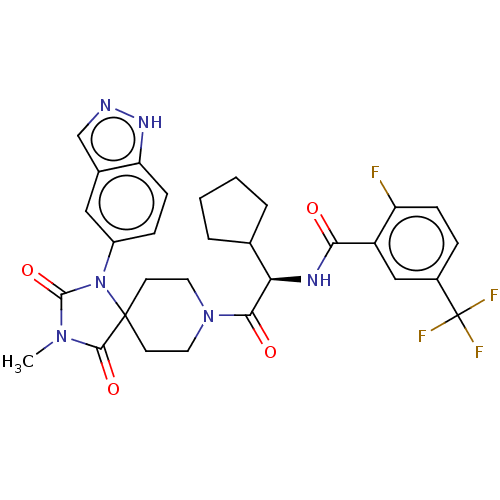 ((R)—N-(2-(1-(1H-Indazol-5-yl)-3-methyl-2,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369945 ((R)—N-(2-(1-(1H-Indazol-5-yl)-3-methyl-2,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369946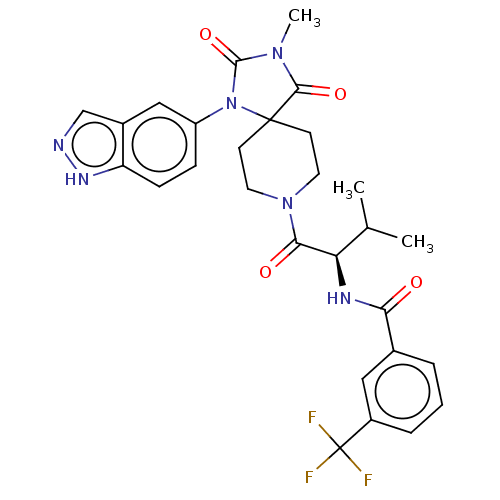 (US10233182, Example 59) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369947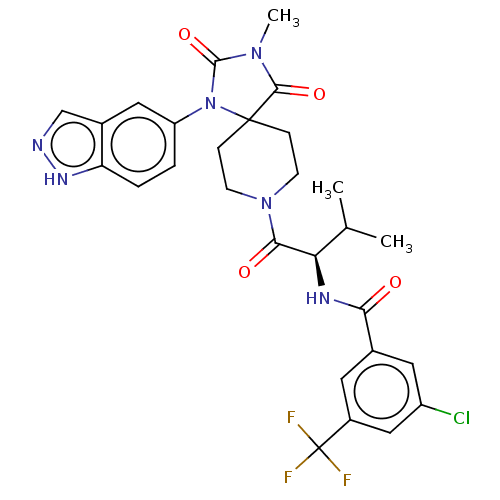 ((R)—N-(1-(1-(1H-Indazol-5-yl)-3-methyl-2,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369948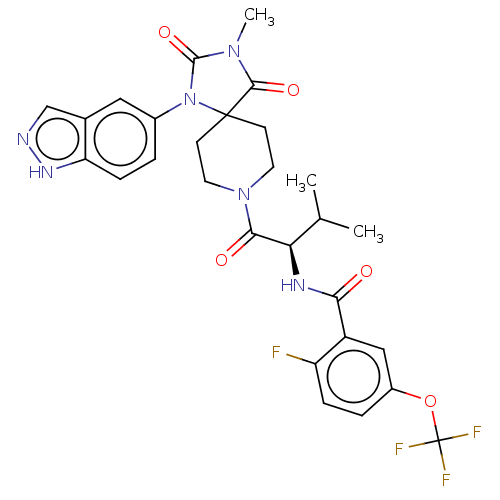 ((R)—N-(1-(1-(1H-Indazol-5-yl)-3-methyl-2,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369949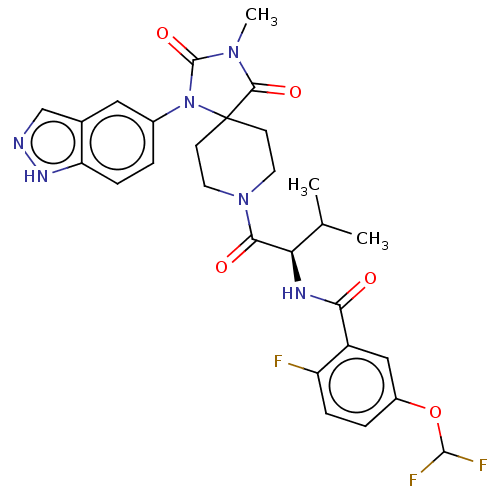 ((R)—N-(1-(1-(1H-Indazol-5-yl)-3-methyl-2,4-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369950 ((R)-2-Fluoro-N-(3-methyl-1-(3-methyl-1-(4-(oxetan-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369951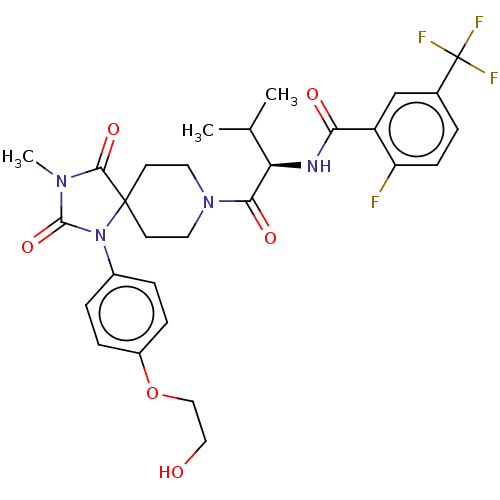 ((R)-2-Fluoro-N-(1-(1-(4-(2-hydroxyethoxy)phenyl)-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369952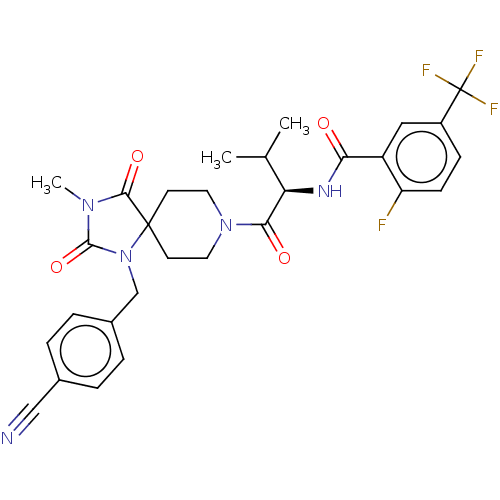 ((R)—N-(1-(1-(4-Cyanobenzyl)-3-methyl-2,4-diox...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369953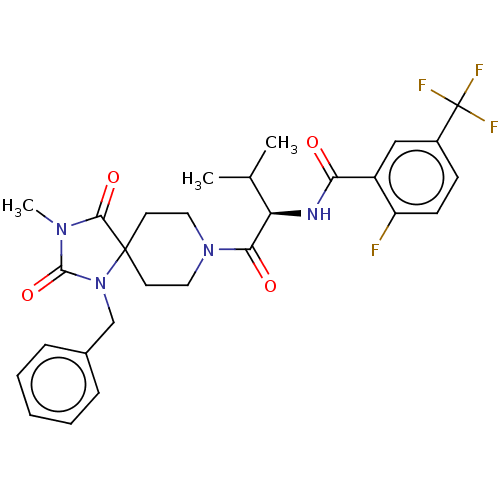 ((R)—N-(1-(1-Benzyl-3-methyl-2,4-dioxo-1,3,8-t...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369954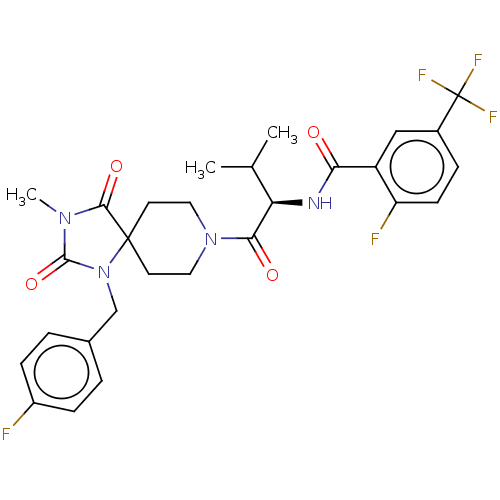 ((R)-2-Fluoro-N-(1-(1-(4-fluorobenzyl)-3-methyl-2,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369955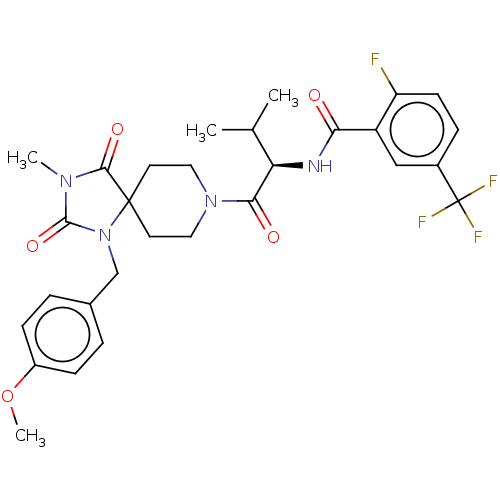 ((R)-2-Fluoro-N-(1-(1-(4-methoxybenzyl)-3-methyl-2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369956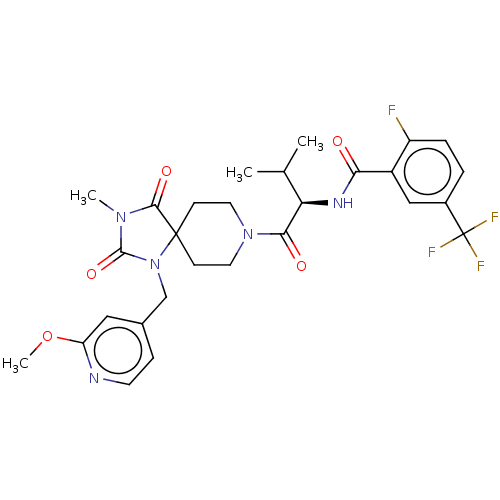 ((R)-2-Fluoro-N-(1-(1-((2-methoxypyridin-4-yl)methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM369957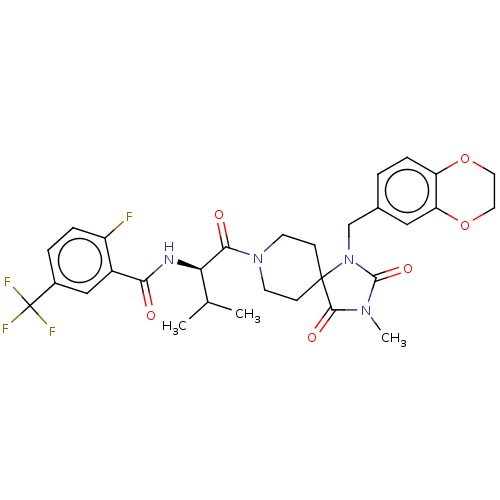 ((R)—N-(1-(1-((2,3-Dihydrobenzo[b][1,4]dioxin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono | Assay Description The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... | J Med Chem 51: 2227-2243 (2008) BindingDB Entry DOI: 10.7270/Q2QF8W5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 245 total ) | Next | Last >> |