 Found 15 hits for monomerid = 369940
Found 15 hits for monomerid = 369940 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM369940
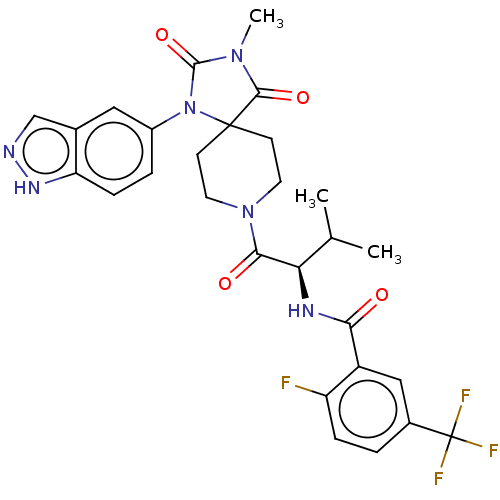
((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono
| Assay Description
The efficacy of the Examples described herein, as inhibitors of ATX are demonstrated and confirmed by pharmacological in vitro assays. The following ... |
J Med Chem 51: 2227-2243 (2008)
BindingDB Entry DOI: 10.7270/Q2QF8W5N |
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1/3
(Homo sapiens (Human)) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LPAR3 (unknown origin) |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Mus musculus) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in mouse plasma assessed as reduction in LPA (18:2) production incubated for 2 hrs LC-MS-/MS analysis |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of autotaxin in human plasma assessed as reduction in LPA (18:2) production incubated for 2 hrs LC-MS-/MS analysis |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GLS2
(Saccharomyces cerevisiae) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by manual patch clamp assay |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GLS2
(Saccharomyces cerevisiae) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by GLP based assay |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of ENPP1 (unknown origin) |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor 1
(Homo sapiens (Human)) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LPAR1 (unknown origin) |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
Lysophosphatidic acid receptor Edg-4
(Homo sapiens (Human)) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LPAR2 (unknown origin) |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2
(Homo sapiens (Human)) | BDBM369940

((R)—N-(1-(1-(1H-indazol-5-yl)-3-methyl-2,4-di...)Show SMILES CC(C)[C@@H](NC(=O)c1cc(ccc1F)C(F)(F)F)C(=O)N1CCC2(CC1)N(C(=O)N(C)C2=O)c1ccc2[nH]ncc2c1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal FLAG-tagged human autotaxin expressed in Freestyle 293 cells assessed as reduction in choline release from LPC 16:0 pre-incu... |
J Med Chem 63: 7840-7856 (2020)
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data