

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246570 (CHEMBL4081509) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of organophosphate inhibited human erythrocyte AChE assessed as organophosphate IC50 using acetylthiocholine iodide as substrate preincu... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246567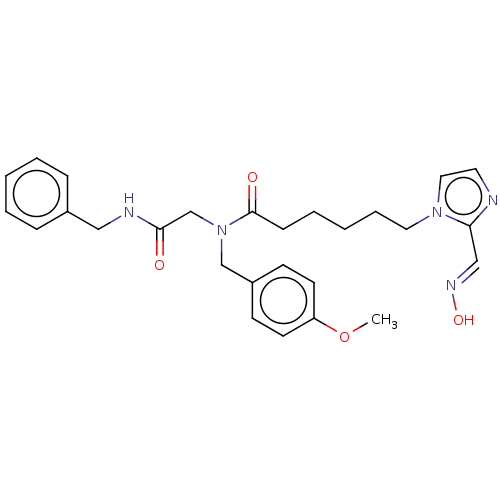 (CHEMBL4063984) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of organophosphate inhibited human erythrocyte AChE assessed as organophosphate IC50 using acetylthiocholine iodide as substrate preincu... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246564 (CHEMBL4091748) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of organophosphate inhibited human erythrocyte AChE assessed as organophosphate IC50 using acetylthiocholine iodide as substrate preincu... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246568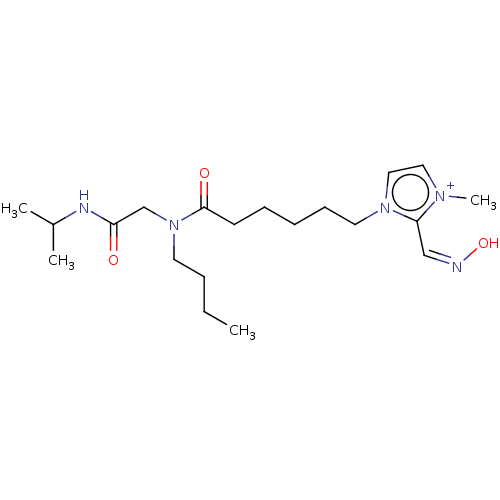 (CHEMBL4096126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of organophosphate inhibited human erythrocyte AChE assessed as organophosphate IC50 using acetylthiocholine iodide as substrate preincu... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246602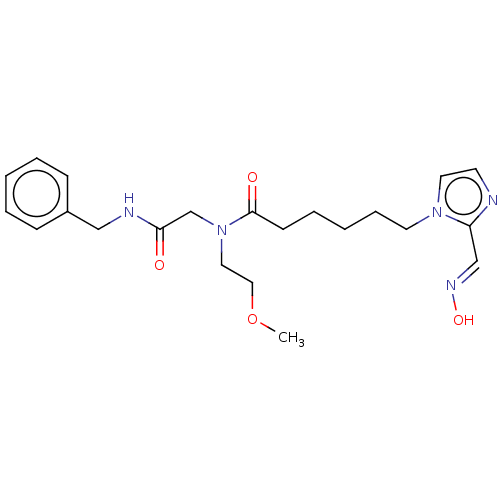 (CHEMBL4072579) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of organophosphate inhibited human erythrocyte AChE assessed as organophosphate IC50 using acetylthiocholine iodide as substrate preincu... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246585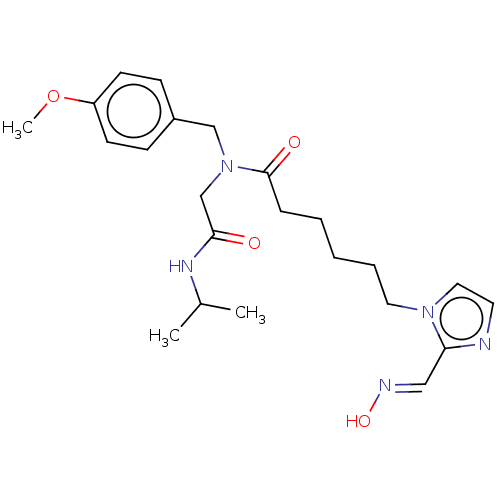 (CHEMBL4090842) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of organophosphate inhibited human erythrocyte AChE assessed as organophosphate IC50 using acetylthiocholine iodide as substrate preincu... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246565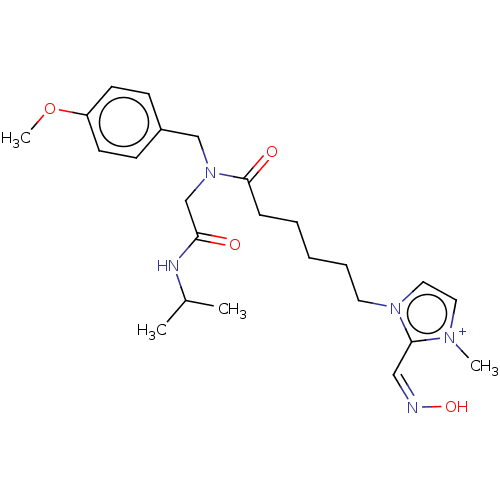 (CHEMBL4068829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of organophosphate inhibited human erythrocyte AChE assessed as organophosphate IC50 using acetylthiocholine iodide as substrate preincu... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246558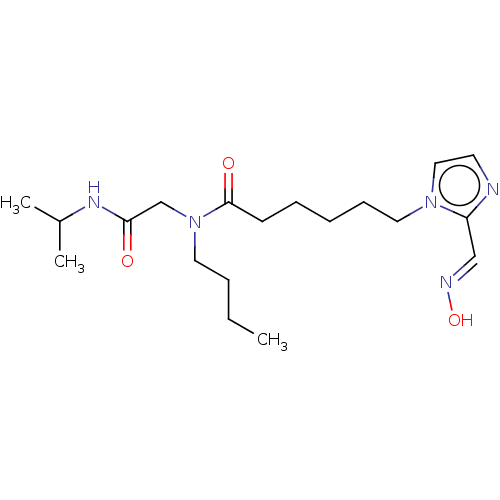 (CHEMBL4069847) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of organophosphate inhibited human erythrocyte AChE assessed as organophosphate IC50 using acetylthiocholine iodide as substrate preincu... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246606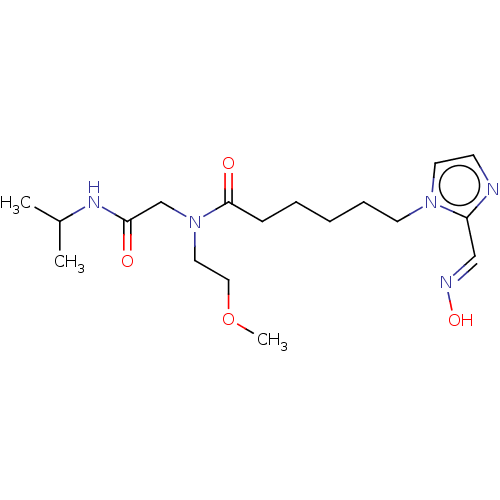 (CHEMBL4099486) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of organophosphate inhibited human erythrocyte AChE assessed as organophosphate IC50 using acetylthiocholine iodide as substrate preincu... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246559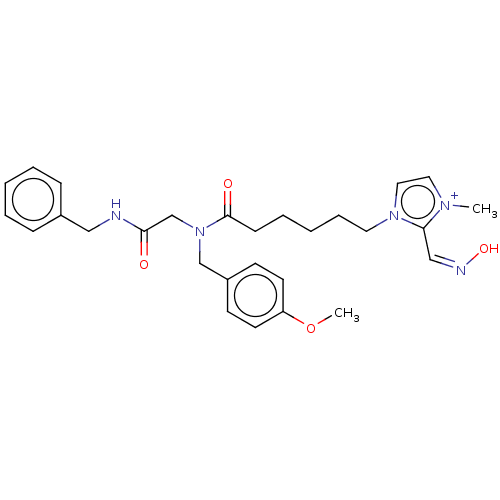 (CHEMBL4059560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of organophosphate inhibited human erythrocyte AChE assessed as organophosphate IC50 using acetylthiocholine iodide as substrate preincu... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246565 (CHEMBL4068829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.20E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246568 (CHEMBL4096126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.13E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246570 (CHEMBL4081509) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.52E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50011780 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.76E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50333779 (1,3-Bis(4-(Hydroxyimino-methyl)-pyridinium)-2-oxap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 3.13E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246558 (CHEMBL4069847) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.14E+6 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246567 (CHEMBL4063984) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.74E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246564 (CHEMBL4091748) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.29E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246606 (CHEMBL4099486) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.47E+6 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246565 (CHEMBL4068829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.90E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246559 (CHEMBL4059560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246570 (CHEMBL4081509) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.22E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50011780 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.16E+6 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246568 (CHEMBL4096126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.21E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of tabun inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50011780 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7.06E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of tabun inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50333779 (1,3-Bis(4-(Hydroxyimino-methyl)-pyridinium)-2-oxap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 9.73E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of tabun inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246559 (CHEMBL4059560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246566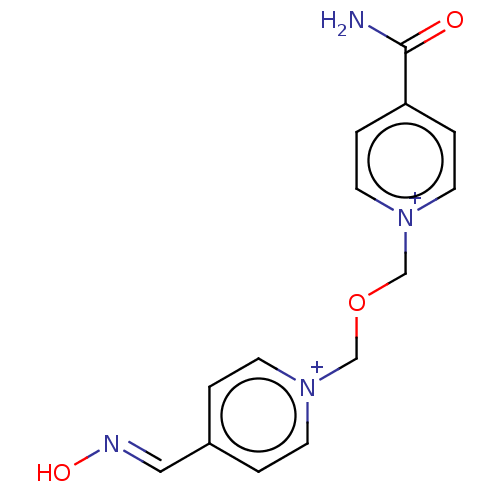 (CHEMBL4079076) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246602 (CHEMBL4072579) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 7.82E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246567 (CHEMBL4063984) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.55E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246567 (CHEMBL4063984) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of VX inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate addi... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50011780 (2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.81E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of VX inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate addi... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246606 (CHEMBL4099486) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.85E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246568 (CHEMBL4096126) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.67E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50333779 (1,3-Bis(4-(Hydroxyimino-methyl)-pyridinium)-2-oxap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 9.46E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246570 (CHEMBL4081509) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of tabun inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246566 (CHEMBL4079076) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 4.72E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of cyclosarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substr... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246602 (CHEMBL4072579) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.66E+6 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246585 (CHEMBL4090842) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 5.27E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246564 (CHEMBL4091748) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.20E+6 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246566 (CHEMBL4079076) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of VX inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate addi... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50333779 (1,3-Bis(4-(Hydroxyimino-methyl)-pyridinium)-2-oxap...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of VX inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate addi... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246559 (CHEMBL4059560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of VX inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate addi... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246565 (CHEMBL4068829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.16E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of VX inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate addi... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246564 (CHEMBL4091748) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 8.16E+5 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of VX inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate addi... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246565 (CHEMBL4068829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of tabun inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246558 (CHEMBL4069847) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.96E+6 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of sarin inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50246559 (CHEMBL4059560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a |
TNO Curated by ChEMBL | Assay Description Reactivation of tabun inhibited human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated up to 60 mins followed by substrate a... | J Med Chem 60: 9376-9392 (2017) Article DOI: 10.1021/acs.jmedchem.7b01083 BindingDB Entry DOI: 10.7270/Q27W6FMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||