 Found 90 hits Enz. Inhib. hit(s) with all data for entry = 50001739
Found 90 hits Enz. Inhib. hit(s) with all data for entry = 50001739 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50341410
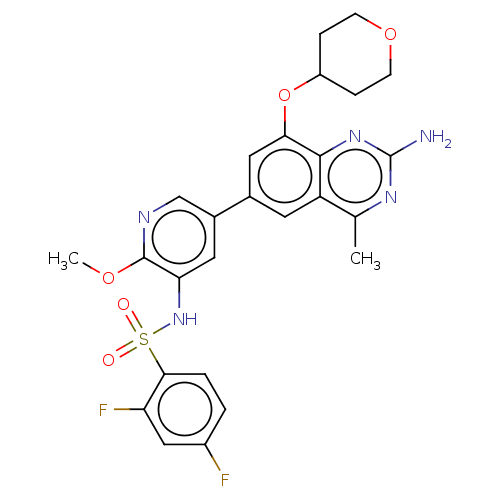
(CHEMBL4166144 | US11534443, Example 1)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-9-15(11-22(24(19)32-26(29)31-14)38-18-5-7-37-8-6-18)16-10-21(25(36-2)30-13-16)33-39(34,35)23-4-3-17(27)12-20(23)28/h3-4,9-13,18,33H,5-8H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50341409
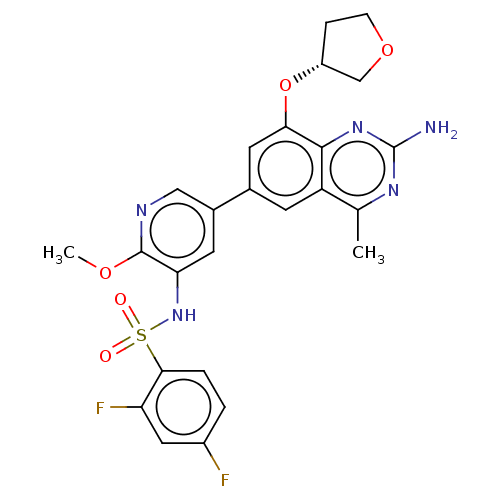
(CHEMBL4176771 | US11534443, Example 9)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(O[C@@H]2CCOC2)c2nc(N)nc(C)c2c1 |r| Show InChI InChI=1S/C25H23F2N5O5S/c1-13-18-7-14(9-21(23(18)31-25(28)30-13)37-17-5-6-36-12-17)15-8-20(24(35-2)29-11-15)32-38(33,34)22-4-3-16(26)10-19(22)27/h3-4,7-11,17,32H,5-6,12H2,1-2H3,(H2,28,30,31)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50341408

(CHEMBL4166594 | US11534443, Example 29)Show SMILES CNc1nc(C)c2cc(cc(OC3CCOCC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C27H27F2N5O5S/c1-15-20-10-16(12-23(25(20)33-27(30-2)32-15)39-19-6-8-38-9-7-19)17-11-22(26(37-3)31-14-17)34-40(35,36)24-5-4-18(28)13-21(24)29/h4-5,10-14,19,34H,6-9H2,1-3H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50341416
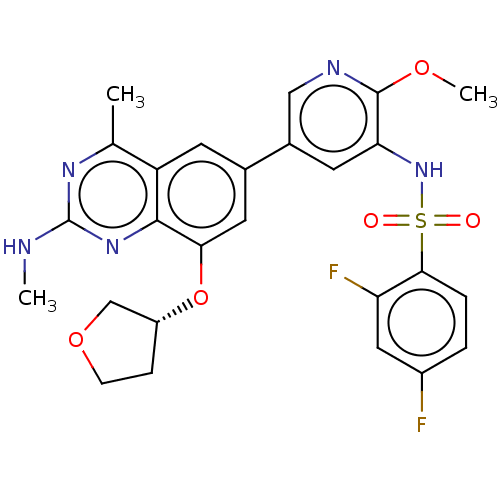
(CHEMBL4174909 | US11534443, Example 32)Show SMILES CNc1nc(C)c2cc(cc(O[C@@H]3CCOC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 |r| Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-8-15(10-22(38-18-6-7-37-13-18)24(19)32-26(29-2)31-14)16-9-21(25(36-3)30-12-16)33-39(34,35)23-5-4-17(27)11-20(23)28/h4-5,8-12,18,33H,6-7,13H2,1-3H3,(H,29,31,32)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341409

(CHEMBL4176771 | US11534443, Example 9)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(O[C@@H]2CCOC2)c2nc(N)nc(C)c2c1 |r| Show InChI InChI=1S/C25H23F2N5O5S/c1-13-18-7-14(9-21(23(18)31-25(28)30-13)37-17-5-6-36-12-17)15-8-20(24(35-2)29-11-15)32-38(33,34)22-4-3-16(26)10-19(22)27/h3-4,7-11,17,32H,5-6,12H2,1-2H3,(H2,28,30,31)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341410

(CHEMBL4166144 | US11534443, Example 1)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-9-15(11-22(24(19)32-26(29)31-14)38-18-5-7-37-8-6-18)16-10-21(25(36-2)30-13-16)33-39(34,35)23-4-3-17(27)12-20(23)28/h3-4,9-13,18,33H,5-8H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341418
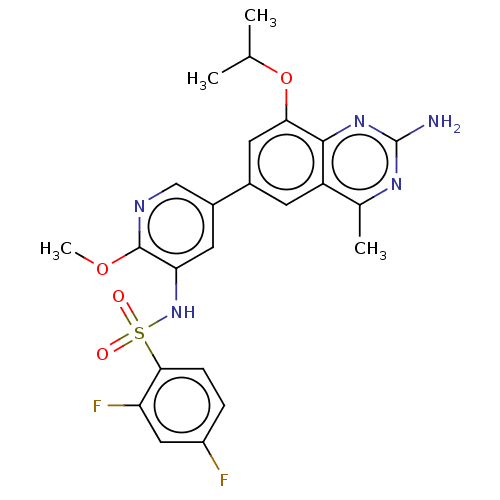
(CHEMBL4174456 | US11534443, Example 26)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC(C)C)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C24H23F2N5O4S/c1-12(2)35-20-9-14(7-17-13(3)29-24(27)30-22(17)20)15-8-19(23(34-4)28-11-15)31-36(32,33)21-6-5-16(25)10-18(21)26/h5-12,31H,1-4H3,(H2,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341424
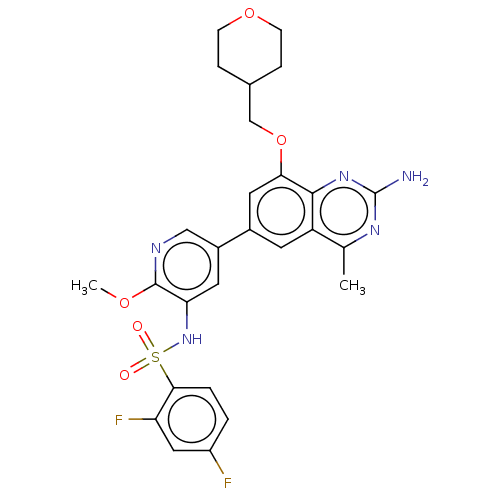
(CHEMBL4165063 | US11534443, Example 13)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OCC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C27H27F2N5O5S/c1-15-20-9-17(11-23(25(20)33-27(30)32-15)39-14-16-5-7-38-8-6-16)18-10-22(26(37-2)31-13-18)34-40(35,36)24-4-3-19(28)12-21(24)29/h3-4,9-13,16,34H,5-8,14H2,1-2H3,(H2,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341410

(CHEMBL4166144 | US11534443, Example 1)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-9-15(11-22(24(19)32-26(29)31-14)38-18-5-7-37-8-6-18)16-10-21(25(36-2)30-13-16)33-39(34,35)23-4-3-17(27)12-20(23)28/h3-4,9-13,18,33H,5-8H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342400

(CHEMBL4169573 | US11534443, Example 11)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(O[C@H]2CCOC2)c2nc(N)nc(C)c2c1 |r| Show InChI InChI=1S/C25H23F2N5O5S/c1-13-18-7-14(9-21(23(18)31-25(28)30-13)37-17-5-6-36-12-17)15-8-20(24(35-2)29-11-15)32-38(33,34)22-4-3-16(26)10-19(22)27/h3-4,7-11,17,32H,5-6,12H2,1-2H3,(H2,28,30,31)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342401
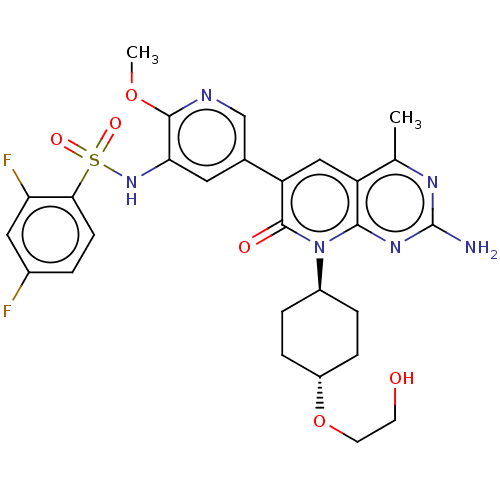
(CHEMBL4166142)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:34.40,wD:31.33,(28.85,-30.23,;28.85,-28.69,;27.51,-27.92,;26.18,-28.69,;24.84,-27.92,;24.84,-26.38,;26.17,-25.61,;27.51,-26.37,;28.84,-25.6,;30.18,-26.38,;30.96,-27.71,;29.1,-27.48,;31.51,-25.6,;31.5,-24.06,;32.83,-23.28,;34.16,-24.05,;35.5,-23.27,;34.17,-25.59,;32.85,-26.36,;32.86,-27.9,;23.5,-25.61,;22.17,-26.39,;20.84,-25.62,;19.51,-26.4,;19.51,-27.93,;18.17,-25.63,;18.17,-24.09,;16.83,-23.32,;19.5,-23.31,;20.84,-24.08,;22.17,-23.31,;22.16,-21.77,;23.5,-20.99,;23.49,-19.45,;22.16,-18.69,;20.83,-19.46,;20.83,-21,;22.15,-17.15,;20.82,-16.38,;20.82,-14.84,;19.48,-14.07,;23.5,-24.08,;24.84,-23.31,)| Show InChI InChI=1S/C28H30F2N6O6S/c1-15-20-13-21(27(38)36(25(20)34-28(31)33-15)18-4-6-19(7-5-18)42-10-9-37)16-11-23(26(41-2)32-14-16)35-43(39,40)24-8-3-17(29)12-22(24)30/h3,8,11-14,18-19,35,37H,4-7,9-10H2,1-2H3,(H2,31,33,34)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50341416

(CHEMBL4174909 | US11534443, Example 32)Show SMILES CNc1nc(C)c2cc(cc(O[C@@H]3CCOC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 |r| Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-8-15(10-22(38-18-6-7-37-13-18)24(19)32-26(29-2)31-14)16-9-21(25(36-3)30-12-16)33-39(34,35)23-5-4-17(27)11-20(23)28/h4-5,8-12,18,33H,6-7,13H2,1-3H3,(H,29,31,32)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341415
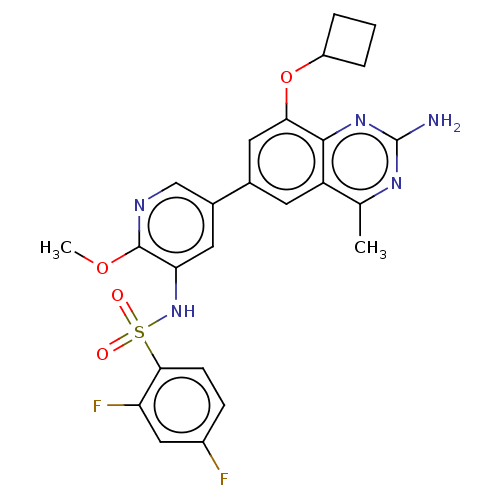
(CHEMBL4168480 | US11534443, Example 24)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C25H23F2N5O4S/c1-13-18-8-14(10-21(36-17-4-3-5-17)23(18)31-25(28)30-13)15-9-20(24(35-2)29-12-15)32-37(33,34)22-7-6-16(26)11-19(22)27/h6-12,17,32H,3-5H2,1-2H3,(H2,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341431
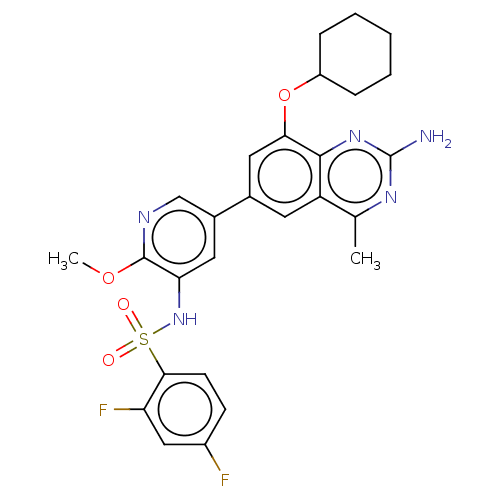
(CHEMBL4162701 | US11534443, Example 22)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCCCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C27H27F2N5O4S/c1-15-20-10-16(12-23(25(20)33-27(30)32-15)38-19-6-4-3-5-7-19)17-11-22(26(37-2)31-14-17)34-39(35,36)24-9-8-18(28)13-21(24)29/h8-14,19,34H,3-7H2,1-2H3,(H2,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341416

(CHEMBL4174909 | US11534443, Example 32)Show SMILES CNc1nc(C)c2cc(cc(O[C@@H]3CCOC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 |r| Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-8-15(10-22(38-18-6-7-37-13-18)24(19)32-26(29-2)31-14)16-9-21(25(36-3)30-12-16)33-39(34,35)23-5-4-17(27)11-20(23)28/h4-5,8-12,18,33H,6-7,13H2,1-3H3,(H,29,31,32)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341409

(CHEMBL4176771 | US11534443, Example 9)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(O[C@@H]2CCOC2)c2nc(N)nc(C)c2c1 |r| Show InChI InChI=1S/C25H23F2N5O5S/c1-13-18-7-14(9-21(23(18)31-25(28)30-13)37-17-5-6-36-12-17)15-8-20(24(35-2)29-11-15)32-38(33,34)22-4-3-16(26)10-19(22)27/h3-4,7-11,17,32H,5-6,12H2,1-2H3,(H2,28,30,31)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341419
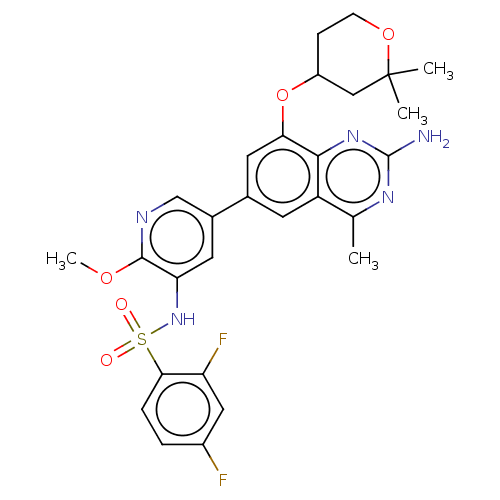
(CHEMBL4172954 | US11534443, Example 19)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOC(C)(C)C2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C28H29F2N5O5S/c1-15-20-9-16(11-23(25(20)34-27(31)33-15)40-19-7-8-39-28(2,3)13-19)17-10-22(26(38-4)32-14-17)35-41(36,37)24-6-5-18(29)12-21(24)30/h5-6,9-12,14,19,35H,7-8,13H2,1-4H3,(H2,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341416

(CHEMBL4174909 | US11534443, Example 32)Show SMILES CNc1nc(C)c2cc(cc(O[C@@H]3CCOC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 |r| Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-8-15(10-22(38-18-6-7-37-13-18)24(19)32-26(29-2)31-14)16-9-21(25(36-3)30-12-16)33-39(34,35)23-5-4-17(27)11-20(23)28/h4-5,8-12,18,33H,6-7,13H2,1-3H3,(H,29,31,32)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341414

(CHEMBL4176338 | US11534443, Example 23)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H25F2N5O4S/c1-14-19-9-15(11-22(24(19)32-26(29)31-14)37-18-5-3-4-6-18)16-10-21(25(36-2)30-13-16)33-38(34,35)23-8-7-17(27)12-20(23)28/h7-13,18,33H,3-6H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341429
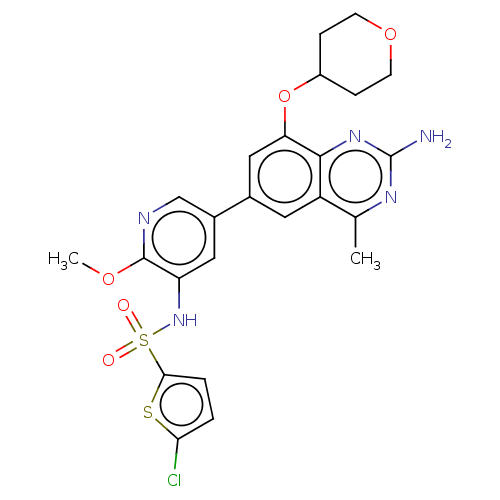
(CHEMBL4175228 | US11534443, Example 4)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(Cl)s1)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C24H24ClN5O5S2/c1-13-17-9-14(11-19(22(17)29-24(26)28-13)35-16-5-7-34-8-6-16)15-10-18(23(33-2)27-12-15)30-37(31,32)21-4-3-20(25)36-21/h3-4,9-12,16,30H,5-8H2,1-2H3,(H2,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342403

(CHEMBL4160104 | US11534443, Example 2)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1Cl)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H25ClFN5O5S/c1-14-19-9-15(11-22(24(19)32-26(29)31-14)38-18-5-7-37-8-6-18)16-10-21(25(36-2)30-13-16)33-39(34,35)23-4-3-17(28)12-20(23)27/h3-4,9-13,18,33H,5-8H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342406

(CHEMBL4167641 | US11534443, Example 20)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OCC2CCCO2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-8-15(10-22(24(19)32-26(29)31-14)38-13-18-4-3-7-37-18)16-9-21(25(36-2)30-12-16)33-39(34,35)23-6-5-17(27)11-20(23)28/h5-6,8-12,18,33H,3-4,7,13H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341423

(CHEMBL4161649 | US11534443, Example 21)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OCC2CCOC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-7-16(9-22(24(19)32-26(29)31-14)38-13-15-5-6-37-12-15)17-8-21(25(36-2)30-11-17)33-39(34,35)23-4-3-18(27)10-20(23)28/h3-4,7-11,15,33H,5-6,12-13H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341412
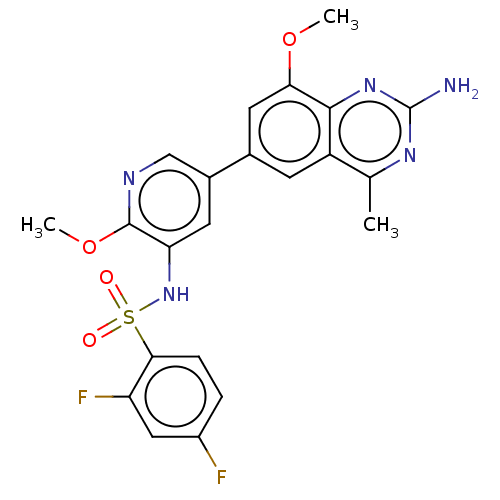
(CHEMBL4166540 | US11534443, Example 28)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C22H19F2N5O4S/c1-11-15-6-12(8-18(32-2)20(15)28-22(25)27-11)13-7-17(21(33-3)26-10-13)29-34(30,31)19-5-4-14(23)9-16(19)24/h4-10,29H,1-3H3,(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341407
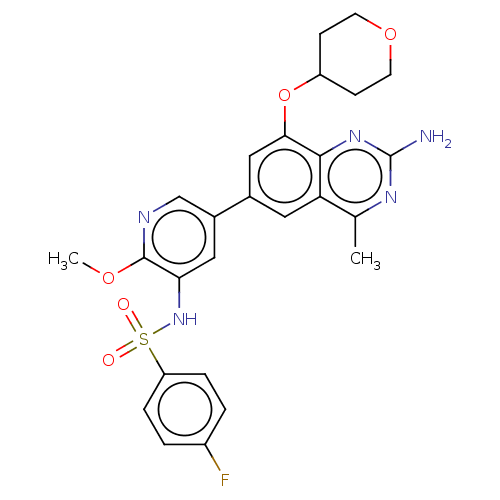
(CHEMBL4167351 | US11534443, Example 3)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H26FN5O5S/c1-15-21-11-16(13-23(24(21)31-26(28)30-15)37-19-7-9-36-10-8-19)17-12-22(25(35-2)29-14-17)32-38(33,34)20-5-3-18(27)4-6-20/h3-6,11-14,19,32H,7-10H2,1-2H3,(H2,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50341408

(CHEMBL4166594 | US11534443, Example 29)Show SMILES CNc1nc(C)c2cc(cc(OC3CCOCC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C27H27F2N5O5S/c1-15-20-10-16(12-23(25(20)33-27(30-2)32-15)39-19-6-8-38-9-7-19)17-11-22(26(37-3)31-14-17)34-40(35,36)24-5-4-18(28)13-21(24)29/h4-5,10-14,19,34H,6-9H2,1-3H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50341409

(CHEMBL4176771 | US11534443, Example 9)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(O[C@@H]2CCOC2)c2nc(N)nc(C)c2c1 |r| Show InChI InChI=1S/C25H23F2N5O5S/c1-13-18-7-14(9-21(23(18)31-25(28)30-13)37-17-5-6-36-12-17)15-8-20(24(35-2)29-11-15)32-38(33,34)22-4-3-16(26)10-19(22)27/h3-4,7-11,17,32H,5-6,12H2,1-2H3,(H2,28,30,31)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50341410

(CHEMBL4166144 | US11534443, Example 1)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-9-15(11-22(24(19)32-26(29)31-14)38-18-5-7-37-8-6-18)16-10-21(25(36-2)30-13-16)33-39(34,35)23-4-3-17(27)12-20(23)28/h3-4,9-13,18,33H,5-8H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342300

(CHEMBL4167026 | US11534443, Example 35)Show SMILES CNc1nc(C)c2cc(cc(O[C@H]3CCOC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 |r| Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-8-15(10-22(38-18-6-7-37-13-18)24(19)32-26(29-2)31-14)16-9-21(25(36-3)30-12-16)33-39(34,35)23-5-4-17(27)11-20(23)28/h4-5,8-12,18,33H,6-7,13H2,1-3H3,(H,29,31,32)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342299

(CHEMBL4169963 | US11534443, Example 27)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OCC2CC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C25H23F2N5O4S/c1-13-18-7-15(9-21(36-12-14-3-4-14)23(18)31-25(28)30-13)16-8-20(24(35-2)29-11-16)32-37(33,34)22-6-5-17(26)10-19(22)27/h5-11,14,32H,3-4,12H2,1-2H3,(H2,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341408

(CHEMBL4166594 | US11534443, Example 29)Show SMILES CNc1nc(C)c2cc(cc(OC3CCOCC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C27H27F2N5O5S/c1-15-20-10-16(12-23(25(20)33-27(30-2)32-15)39-19-6-8-38-9-7-19)17-11-22(26(37-3)31-14-17)34-40(35,36)24-5-4-18(28)13-21(24)29/h4-5,10-14,19,34H,6-9H2,1-3H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342404
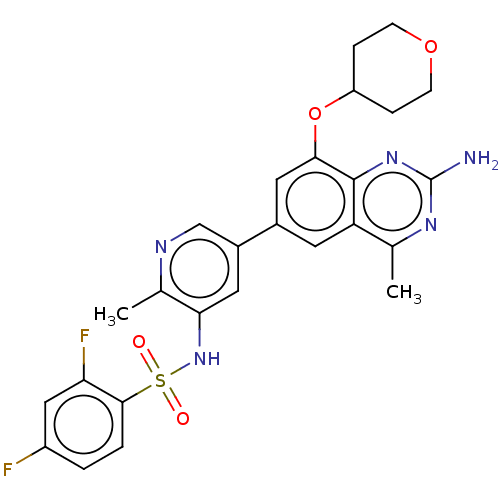
(CHEMBL4168850 | US11534443, Example 7)Show SMILES Cc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H25F2N5O4S/c1-14-20-9-16(11-23(25(20)32-26(29)31-14)37-19-5-7-36-8-6-19)17-10-22(15(2)30-13-17)33-38(34,35)24-4-3-18(27)12-21(24)28/h3-4,9-13,19,33H,5-8H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342399
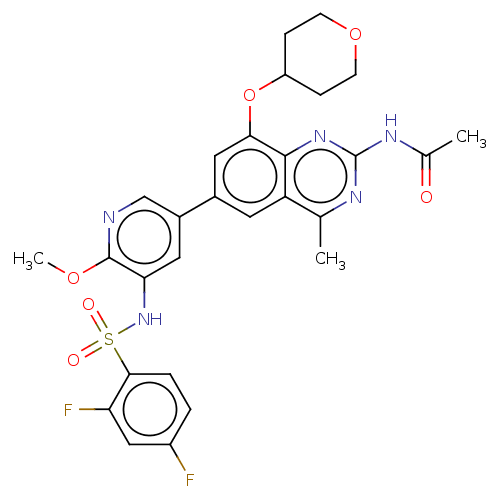
(CHEMBL4159789 | US11534443, Example 42)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOCC2)c2nc(NC(C)=O)nc(C)c2c1 Show InChI InChI=1S/C28H27F2N5O6S/c1-15-21-10-17(12-24(41-20-6-8-40-9-7-20)26(21)34-28(32-15)33-16(2)36)18-11-23(27(39-3)31-14-18)35-42(37,38)25-5-4-19(29)13-22(25)30/h4-5,10-14,20,35H,6-9H2,1-3H3,(H,32,33,34,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342397

(CHEMBL4177219 | US11534443, Example 30)Show SMILES CCNc1nc(C)c2cc(cc(OC3CCOCC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C28H29F2N5O5S/c1-4-31-28-33-16(2)21-11-17(13-24(26(21)34-28)40-20-7-9-39-10-8-20)18-12-23(27(38-3)32-15-18)35-41(36,37)25-6-5-19(29)14-22(25)30/h5-6,11-15,20,35H,4,7-10H2,1-3H3,(H,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342027

(CHEMBL4173411 | US11534443, Example 17)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCC(F)(F)CC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C27H25F4N5O4S/c1-14-19-9-15(11-22(24(19)35-26(32)34-14)40-18-5-7-27(30,31)8-6-18)16-10-21(25(39-2)33-13-16)36-41(37,38)23-4-3-17(28)12-20(23)29/h3-4,9-13,18,36H,5-8H2,1-2H3,(H2,32,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342298
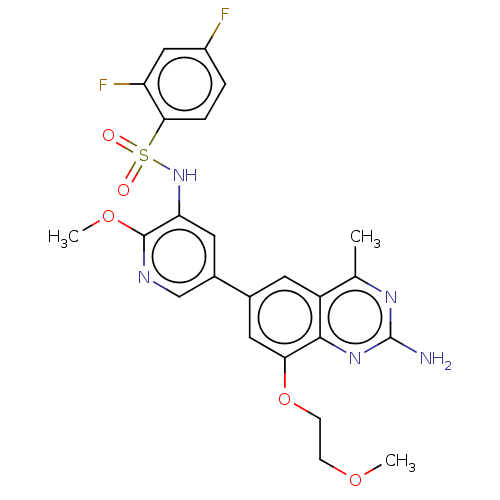
(CHEMBL4159325 | US11534443, Example 25)Show SMILES COCCOc1cc(cc2c(C)nc(N)nc12)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C24H23F2N5O5S/c1-13-17-8-14(10-20(36-7-6-34-2)22(17)30-24(27)29-13)15-9-19(23(35-3)28-12-15)31-37(32,33)21-5-4-16(25)11-18(21)26/h4-5,8-12,31H,6-7H2,1-3H3,(H2,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341430
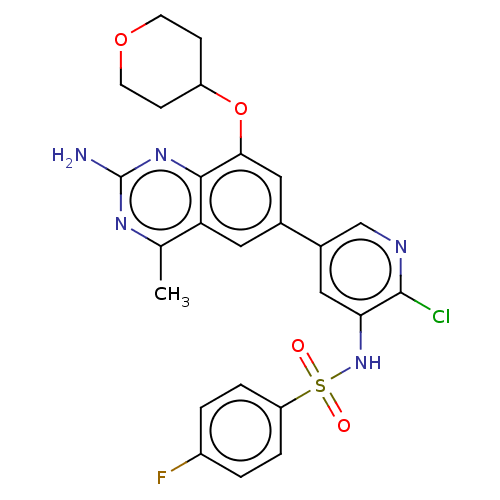
(CHEMBL4176704 | US11534443, Example 8)Show SMILES Cc1nc(N)nc2c(OC3CCOCC3)cc(cc12)-c1cnc(Cl)c(NS(=O)(=O)c2ccc(F)cc2)c1 Show InChI InChI=1S/C25H23ClFN5O4S/c1-14-20-10-15(12-22(23(20)31-25(28)30-14)36-18-6-8-35-9-7-18)16-11-21(24(26)29-13-16)32-37(33,34)19-4-2-17(27)3-5-19/h2-5,10-13,18,32H,6-9H2,1H3,(H2,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50341408

(CHEMBL4166594 | US11534443, Example 29)Show SMILES CNc1nc(C)c2cc(cc(OC3CCOCC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C27H27F2N5O5S/c1-15-20-10-16(12-23(25(20)33-27(30-2)32-15)39-19-6-8-38-9-7-19)17-11-22(26(37-3)31-14-17)34-40(35,36)24-5-4-18(28)13-21(24)29/h4-5,10-14,19,34H,6-9H2,1-3H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2 as substrate by ADP-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341413

(CHEMBL4163575 | US11534443, Example 37)Show SMILES CNc1nc(C)c2cc(cc(OC3CCOC(C)(C)C3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C29H31F2N5O5S/c1-16-21-10-17(12-24(26(21)35-28(32-4)34-16)41-20-8-9-40-29(2,3)14-20)18-11-23(27(39-5)33-15-18)36-42(37,38)25-7-6-19(30)13-22(25)31/h6-7,10-13,15,20,36H,8-9,14H2,1-5H3,(H,32,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341417

(CHEMBL4164634 | US11534443, Example 36)Show SMILES CNc1nc(C)c2cc(cc(OCC3CCOCC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C28H29F2N5O5S/c1-16-21-10-18(12-24(26(21)34-28(31-2)33-16)40-15-17-6-8-39-9-7-17)19-11-23(27(38-3)32-14-19)35-41(36,37)25-5-4-20(29)13-22(25)30/h4-5,10-14,17,35H,6-9,15H2,1-3H3,(H,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341421

(CHEMBL4171472 | US11534443, Example 38)Show SMILES CNc1nc(C)c2cc(cc(OCC3CCOC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C27H27F2N5O5S/c1-15-20-8-17(10-23(25(20)33-27(30-2)32-15)39-14-16-6-7-38-13-16)18-9-22(26(37-3)31-12-18)34-40(35,36)24-5-4-19(28)11-21(24)29/h4-5,8-12,16,34H,6-7,13-14H2,1-3H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341427

(CHEMBL4160524 | US11534443, Example 6)Show SMILES COc1ncc(cc1NS(=O)(=O)C1CC1)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C23H27N5O5S/c1-13-18-9-14(11-20(21(18)27-23(24)26-13)33-16-5-7-32-8-6-16)15-10-19(22(31-2)25-12-15)28-34(29,30)17-3-4-17/h9-12,16-17,28H,3-8H2,1-2H3,(H2,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341406

(CHEMBL4171779 | US11534443, Example 5)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C21H25N5O5S/c1-12-16-8-13(14-9-17(26-32(3,27)28)20(29-2)23-11-14)10-18(19(16)25-21(22)24-12)31-15-4-6-30-7-5-15/h8-11,15,26H,4-7H2,1-3H3,(H2,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341426

(CHEMBL4163911 | US11534443, Example 16)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OCCN2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C27H28F2N6O5S/c1-16-20-11-17(13-23(25(20)33-27(30)32-16)40-10-7-35-5-8-39-9-6-35)18-12-22(26(38-2)31-15-18)34-41(36,37)24-4-3-19(28)14-21(24)29/h3-4,11-15,34H,5-10H2,1-2H3,(H2,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380313
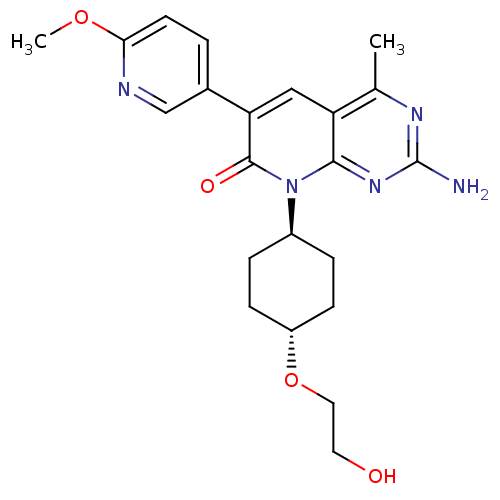
(CHEMBL1234354 | US8633204, 286)Show SMILES COc1ccc(cn1)-c1cc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:19.20,wD:22.27,(7.3,4.56,;5.97,5.33,;4.64,4.56,;3.3,5.33,;1.97,4.56,;1.97,3.02,;3.3,2.25,;4.64,3.02,;.64,2.25,;-.7,3.02,;-2.03,2.25,;-3.37,3.02,;-3.37,4.56,;-4.7,2.25,;-4.7,.71,;-6.03,-.06,;-3.37,-.06,;-2.03,.71,;-.7,-.06,;-.7,-1.6,;.64,-2.37,;.64,-3.91,;-.7,-4.68,;-2.03,-3.91,;-2.03,-2.37,;-.7,-6.22,;.64,-6.99,;.64,-8.53,;1.97,-9.3,;.64,.71,;1.97,-.06,)| Show InChI InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342405
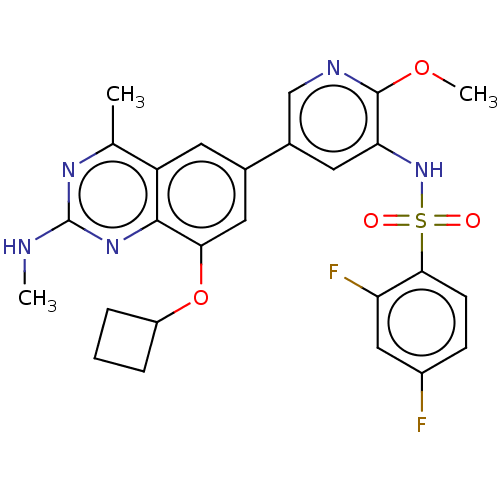
(CHEMBL4172514 | US11534443, Example 41)Show SMILES CNc1nc(C)c2cc(cc(OC3CCC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C26H25F2N5O4S/c1-14-19-9-15(11-22(37-18-5-4-6-18)24(19)32-26(29-2)31-14)16-10-21(25(36-3)30-13-16)33-38(34,35)23-8-7-17(27)12-20(23)28/h7-13,18,33H,4-6H2,1-3H3,(H,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50341425

(CHEMBL4159735 | US11534443, Example 15)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCN(C)CC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C27H28F2N6O4S/c1-15-20-10-16(12-23(25(20)33-27(30)32-15)39-19-6-8-35(2)9-7-19)17-11-22(26(38-3)31-14-17)34-40(36,37)24-5-4-18(28)13-21(24)29/h4-5,10-14,19,34H,6-9H2,1-3H3,(H2,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342398

(CHEMBL4163150 | US11534443, Example 31)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOCC2)c2nc(NCC3CC3)nc(C)c2c1 Show InChI InChI=1S/C30H31F2N5O5S/c1-17-23-11-19(13-26(42-22-7-9-41-10-8-22)28(23)36-30(35-17)34-15-18-3-4-18)20-12-25(29(40-2)33-16-20)37-43(38,39)27-6-5-21(31)14-24(27)32/h5-6,11-14,16,18,22,37H,3-4,7-10,15H2,1-2H3,(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50342378
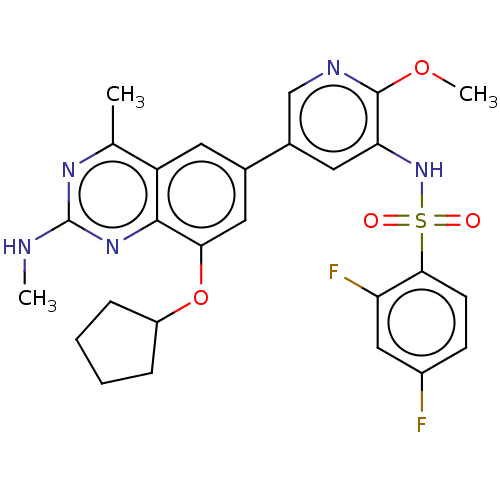
(CHEMBL4168064 | US11534443, Example 40)Show SMILES CNc1nc(C)c2cc(cc(OC3CCCC3)c2n1)-c1cnc(OC)c(NS(=O)(=O)c2ccc(F)cc2F)c1 Show InChI InChI=1S/C27H27F2N5O4S/c1-15-20-10-16(12-23(38-19-6-4-5-7-19)25(20)33-27(30-2)32-15)17-11-22(26(37-3)31-14-17)34-39(35,36)24-9-8-18(28)13-21(24)29/h8-14,19,34H,4-7H2,1-3H3,(H,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2 as substrate by Kinase-Glo assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50341410

(CHEMBL4166144 | US11534443, Example 1)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc(OC2CCOCC2)c2nc(N)nc(C)c2c1 Show InChI InChI=1S/C26H25F2N5O5S/c1-14-19-9-15(11-22(24(19)32-26(29)31-14)38-18-5-7-37-8-6-18)16-10-21(25(36-2)30-13-16)33-39(34,35)23-4-3-17(27)12-20(23)28/h3-4,9-13,18,33H,5-8H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using ULight-4E-BP1 peptide as substrate after 30 mins by Lance Ultra assay |
J Med Chem 61: 6087-6109 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00416
BindingDB Entry DOI: 10.7270/Q28K7CNP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data