

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366784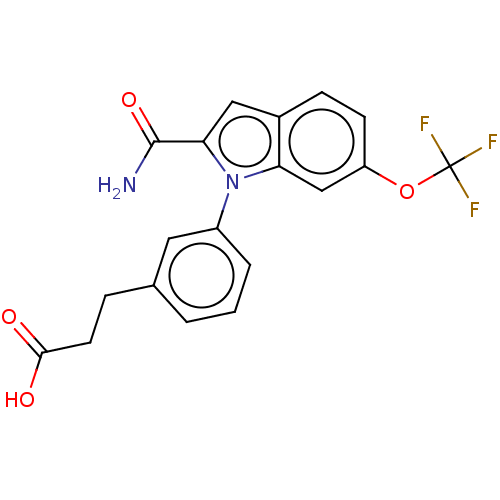 (CHEMBL4171084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366811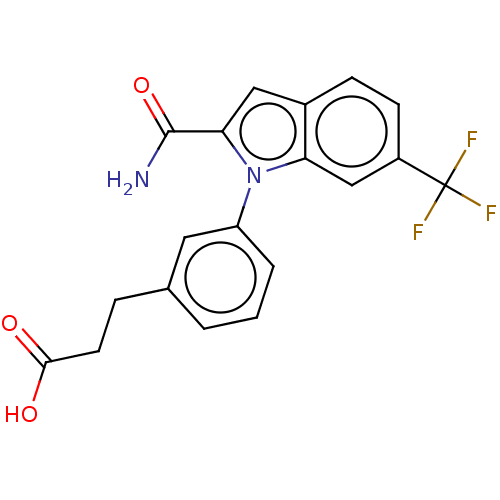 (CHEMBL4176544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366983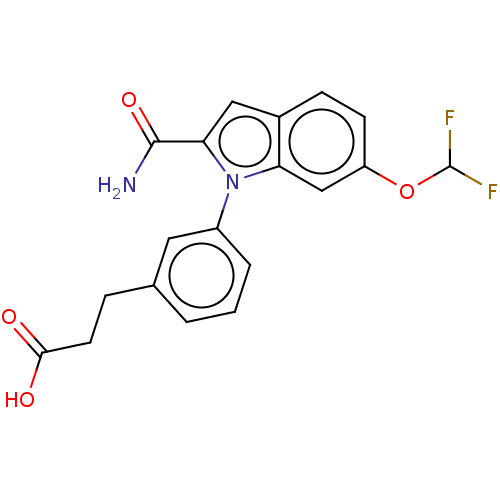 (CHEMBL4160483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366807 (CHEMBL4172222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366839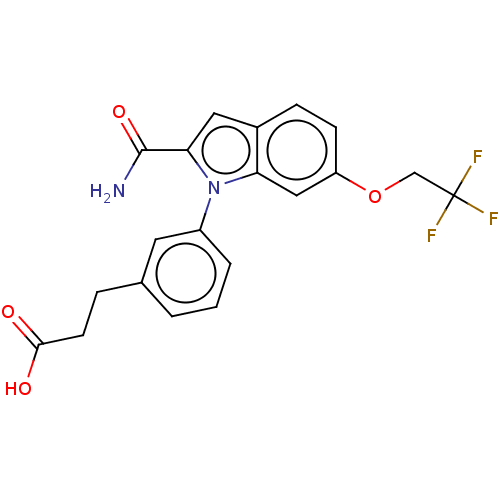 (CHEMBL4175583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Mus musculus) | BDBM50366784 (CHEMBL4171084) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of mouse sPLA2-10 using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substrate additio... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366840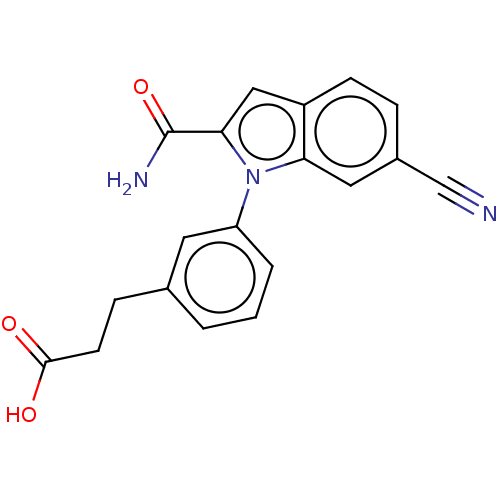 (CHEMBL4174522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-5 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate p... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using HDL as substrate pretreated for 20 mins followed by substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366905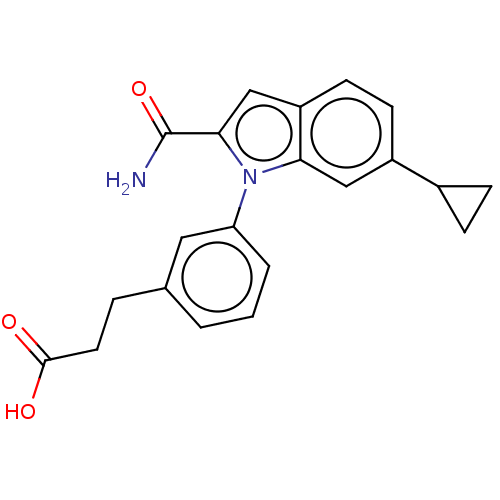 (CHEMBL4173359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366889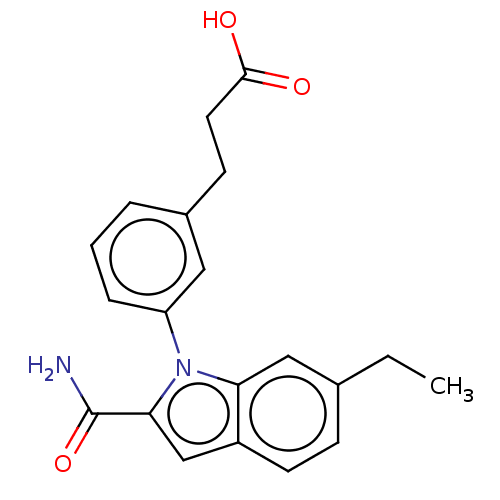 (CHEMBL4175643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366876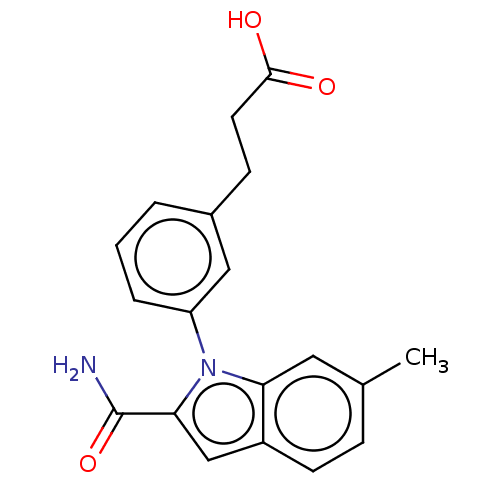 (CHEMBL4171797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366968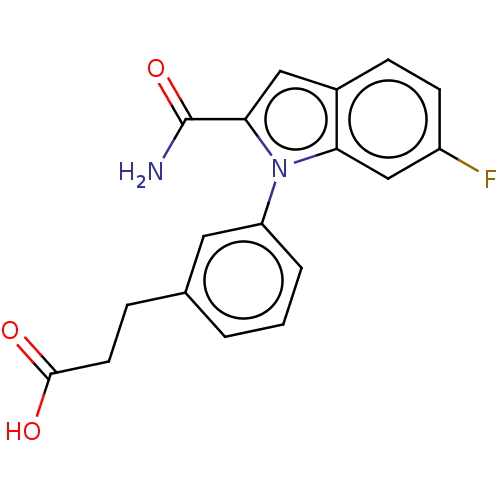 (CHEMBL4161605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366784 (CHEMBL4171084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using HDL as substrate pretreated for 20 mins followed by substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50366784 (CHEMBL4171084) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366983 (CHEMBL4160483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using HDL as substrate pretreated for 20 mins followed by substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366811 (CHEMBL4176544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using HDL as substrate pretreated for 20 mins followed by substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366970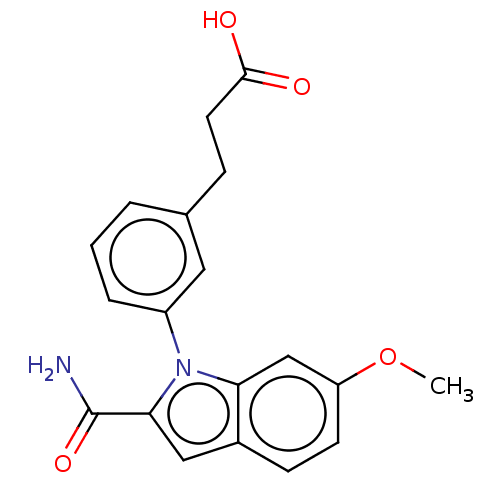 (CHEMBL4160502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366839 (CHEMBL4175583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using HDL as substrate pretreated for 20 mins followed by substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366965 (CHEMBL4159939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50366811 (CHEMBL4176544) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366959 (CHEMBL4176269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366840 (CHEMBL4174522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using HDL as substrate pretreated for 20 mins followed by substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366807 (CHEMBL4172222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using HDL as substrate pretreated for 20 mins followed by substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366783 (CHEMBL4170085) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366905 (CHEMBL4173359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using HDL as substrate pretreated for 20 mins followed by substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50366784 (CHEMBL4171084) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-5 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate p... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50366905 (CHEMBL4173359) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366967 (CHEMBL4168202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366971 (CHEMBL4163859) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50366983 (CHEMBL4160483) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50366811 (CHEMBL4176544) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-5 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate p... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50366840 (CHEMBL4174522) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50366889 (CHEMBL4175643) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366984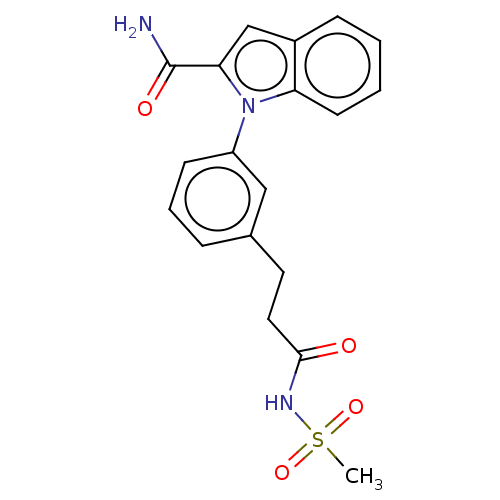 (CHEMBL4172025) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50366807 (CHEMBL4172222) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366804 (CHEMBL4169491) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366857 (CHEMBL4168275) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50366905 (CHEMBL4173359) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-5 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate p... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50366839 (CHEMBL4175583) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366856 (CHEMBL4176688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366964 (CHEMBL4175476) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366960 (CHEMBL4168405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366963 (CHEMBL4160366) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366806 (CHEMBL4166071) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366782 (CHEMBL4164836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366921 (CHEMBL4161571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||