

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M1 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M5 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M2 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M4 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M3 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50374004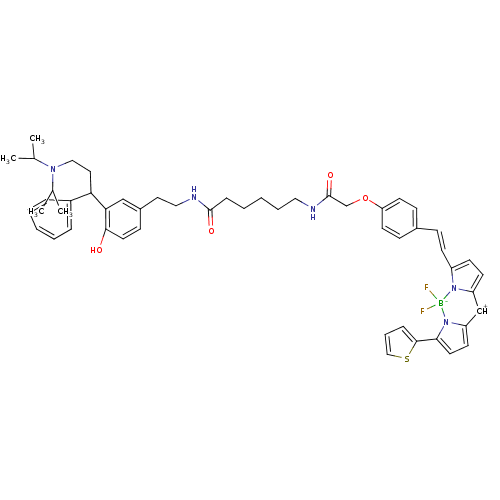 (CHEMBL271108) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M1 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (RAT) | BDBM50374004 (CHEMBL271108) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M4 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50374004 (CHEMBL271108) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M2 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50374004 (CHEMBL271108) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M3 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (RAT) | BDBM50374004 (CHEMBL271108) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor M5 in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50453074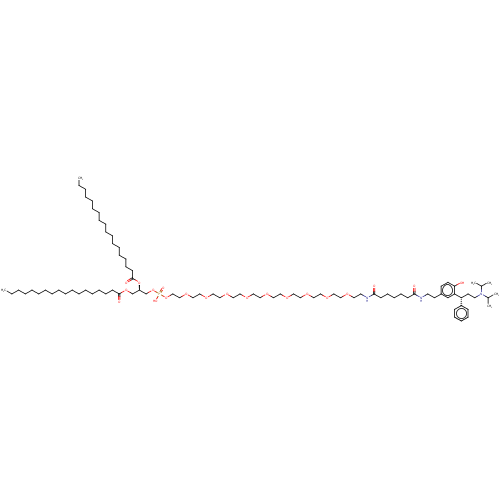 (CHEMBL4215958) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50453077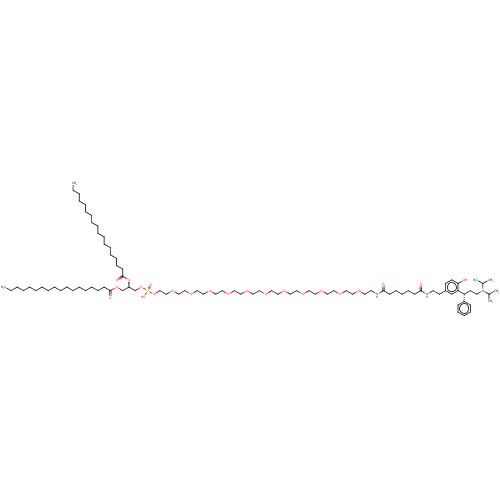 (CHEMBL4216478) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50453073 (CHEMBL4204282) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50453076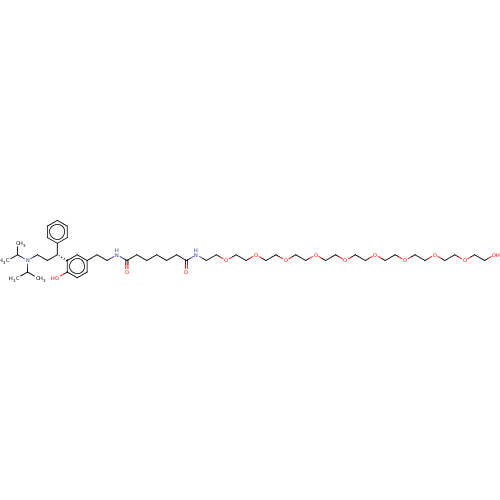 (CHEMBL4211510) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50453068 (CHEMBL4206276) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50453070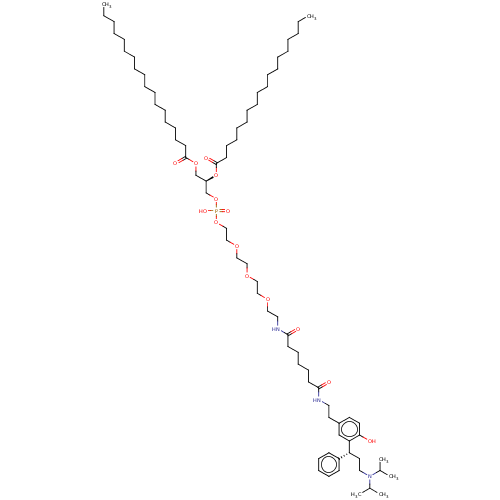 (CHEMBL4208614) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50453072 (CHEMBL4207504) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka Curated by ChEMBL | Assay Description Displacement of [3H]NMS from muscarinic receptor in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method | J Med Chem 61: 4020-4029 (2018) Article DOI: 10.1021/acs.jmedchem.8b00041 BindingDB Entry DOI: 10.7270/Q29Z97H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||