

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50128065 (CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50456340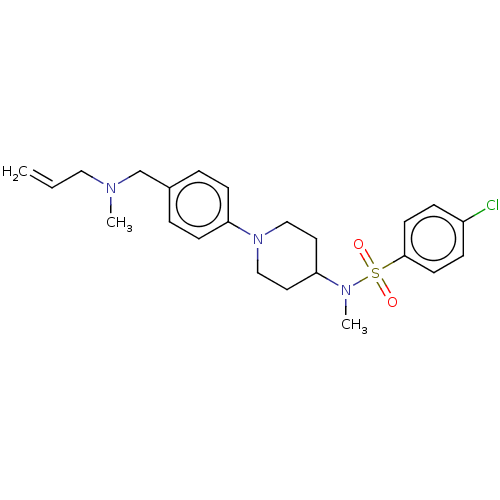 (CHEMBL4207987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50456341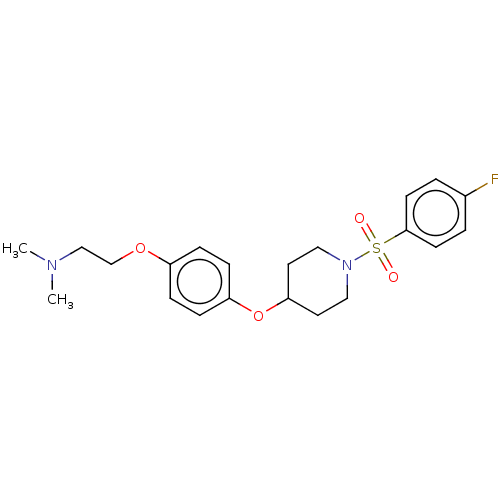 (CHEMBL4212932) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50456342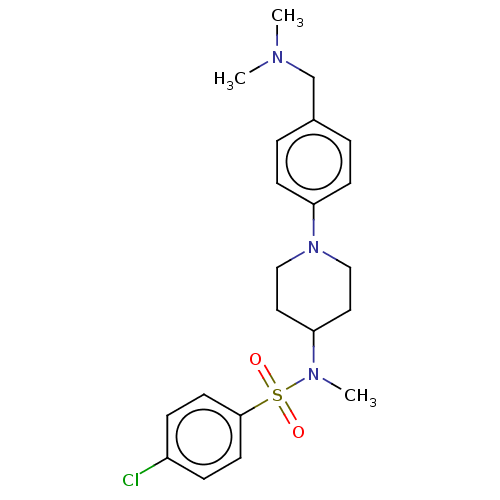 (CHEMBL4210513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST) Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged OSC expressed in Pichia pastoris GS115 using 2,3-oxidosqualene as substrate after 90 mins by 1H NMR method | J Med Chem 61: 5047-5053 (2018) Article DOI: 10.1021/acs.jmedchem.8b00484 BindingDB Entry DOI: 10.7270/Q2NK3HN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||