

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458617 (CHEMBL4215835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458617 (CHEMBL4215835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458614 (CHEMBL4210991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366784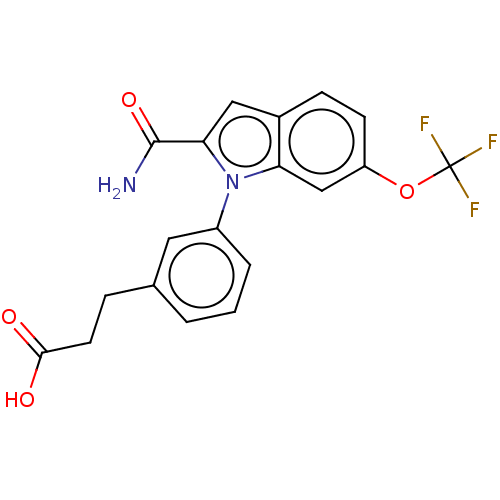 (CHEMBL4171084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458605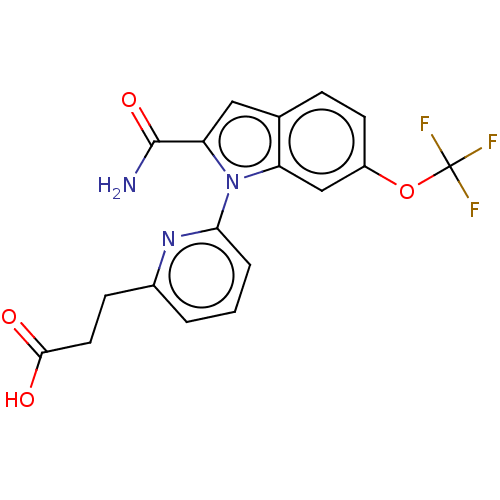 (CHEMBL4217510) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458612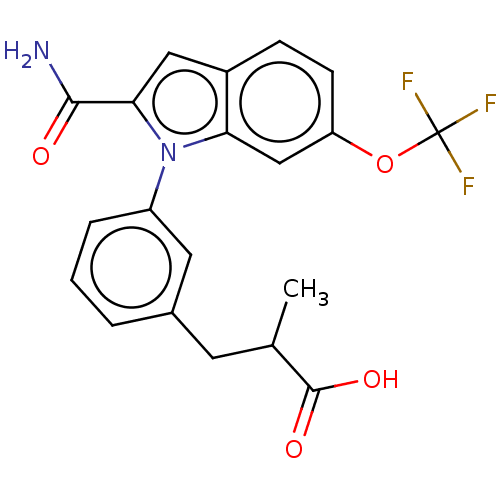 (CHEMBL4214052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458612 (CHEMBL4214052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458613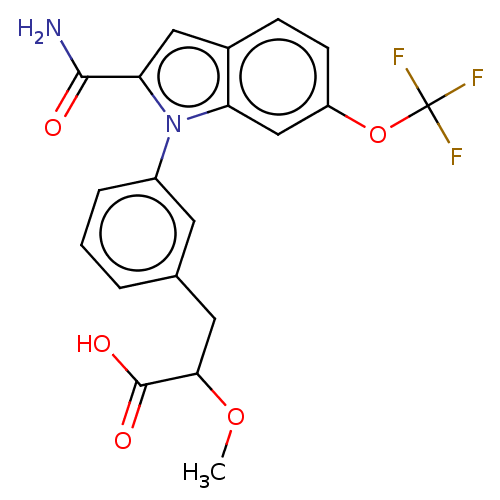 (CHEMBL4204172) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458613 (CHEMBL4204172) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458617 (CHEMBL4215835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 using human HDL as substrate pretreated for 20 mins followed by substrate addition and measured after 60 min... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458608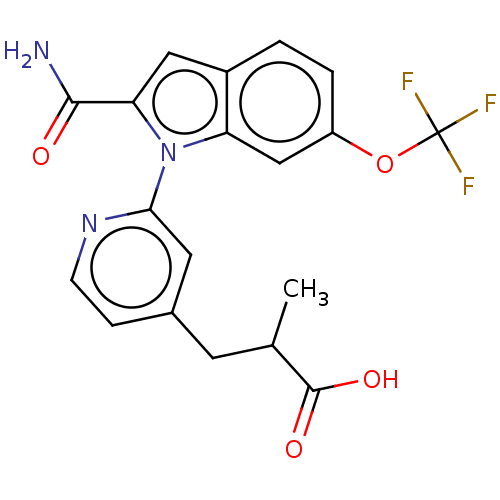 (CHEMBL4205008) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458608 (CHEMBL4205008) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458612 (CHEMBL4214052) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458612 (CHEMBL4214052) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458606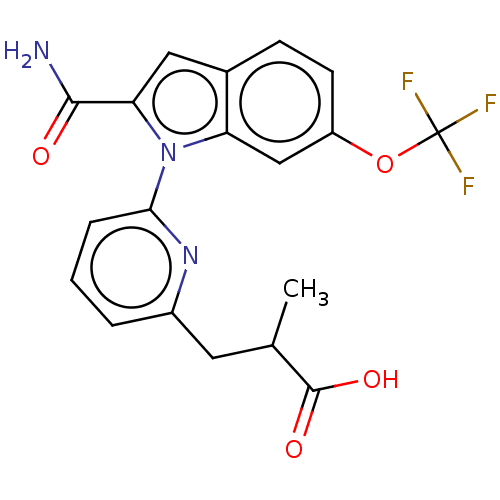 (CHEMBL4205511) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458606 (CHEMBL4205511) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Mus musculus) | BDBM50458617 (CHEMBL4215835) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of mouse sPLA2-10 | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458606 (CHEMBL4205511) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458606 (CHEMBL4205511) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458614 (CHEMBL4210991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 using human HDL as substrate pretreated for 20 mins followed by substrate addition and measured after 60 min... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458609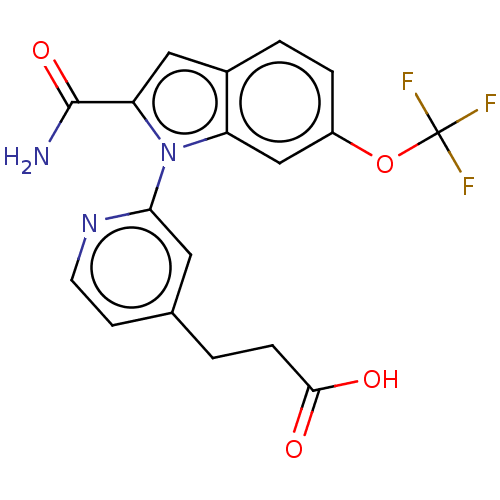 (CHEMBL4213094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458608 (CHEMBL4205008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458608 (CHEMBL4205008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458613 (CHEMBL4204172) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458613 (CHEMBL4204172) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 using human HDL as substrate pretreated for 20 mins followed by substrate addition and measured after 60 min... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458613 (CHEMBL4204172) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458615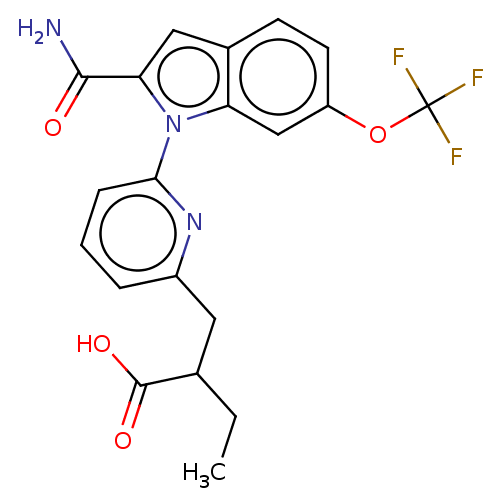 (CHEMBL4203027) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458615 (CHEMBL4203027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458615 (CHEMBL4203027) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458615 (CHEMBL4203027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50458608 (CHEMBL4205008) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50458608 (CHEMBL4205008) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458619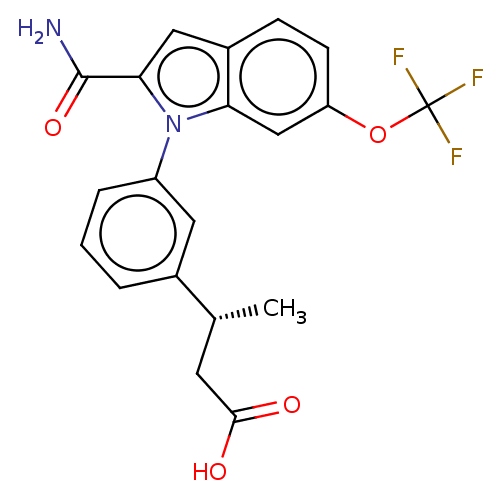 (CHEMBL4207266) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458618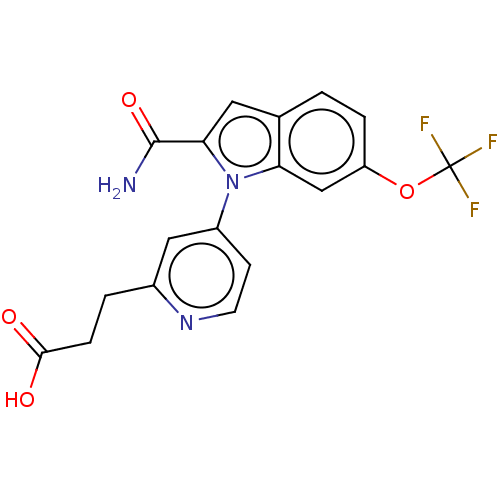 (CHEMBL4206163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458605 (CHEMBL4217510) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 using human HDL as substrate pretreated for 20 mins followed by substrate addition and measured after 60 min... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458606 (CHEMBL4205511) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 using human HDL as substrate pretreated for 20 mins followed by substrate addition and measured after 60 min... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458609 (CHEMBL4213094) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50366784 (CHEMBL4171084) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366784 (CHEMBL4171084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 using human HDL as substrate pretreated for 20 mins followed by substrate addition and measured after 60 min... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458616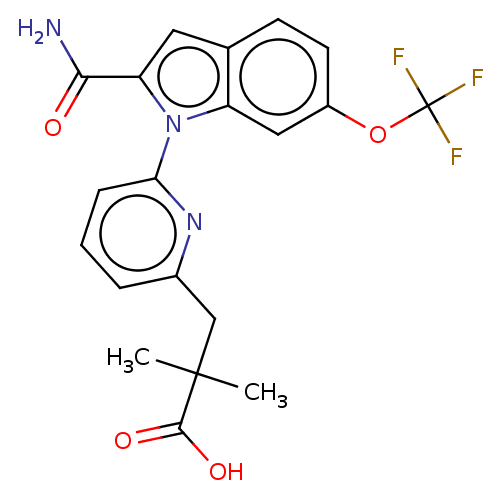 (CHEMBL4204160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458615 (CHEMBL4203027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 using human HDL as substrate pretreated for 20 mins followed by substrate addition and measured after 60 min... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458612 (CHEMBL4214052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 using human HDL as substrate pretreated for 20 mins followed by substrate addition and measured after 60 min... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458612 (CHEMBL4214052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 using human HDL as substrate pretreated for 20 mins followed by substrate addition and measured after 60 min... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458607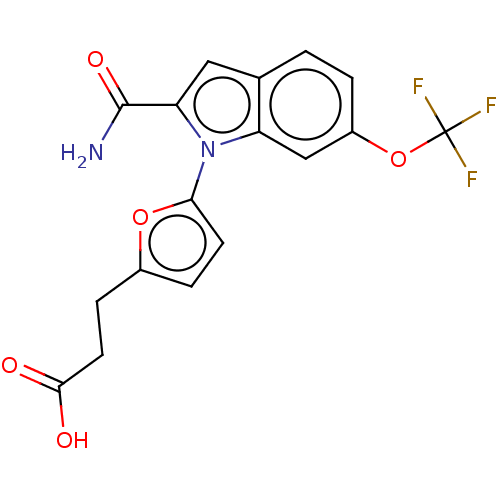 (CHEMBL4207065) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458605 (CHEMBL4217510) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50458606 (CHEMBL4205511) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50458612 (CHEMBL4214052) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-5 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substra... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458617 (CHEMBL4215835) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50458617 (CHEMBL4215835) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-2A (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458609 (CHEMBL4213094) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 using human HDL as substrate pretreated for 20 mins followed by substrate addition and measured after 60 min... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |