

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50130563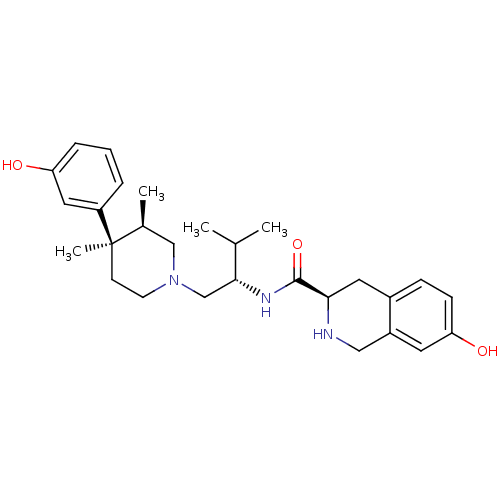 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor (unknown origin) assessed as stimulation of [35S]GTPgammaS | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50026603 (Buprenorphine | CHEBI:3216) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor (unknown origin) assessed as stimulation of [35S]GTPgammaS | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50026614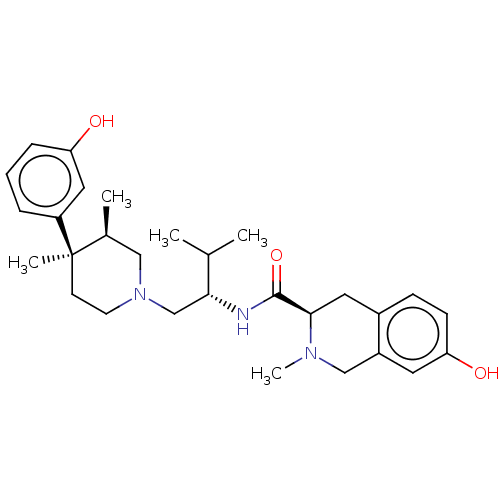 (CHEMBL575508) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50464462 (CHEMBL4283681) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012152 (CHEMBL3264441) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50464462 (CHEMBL4283681) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50464462 (CHEMBL4283681) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50464461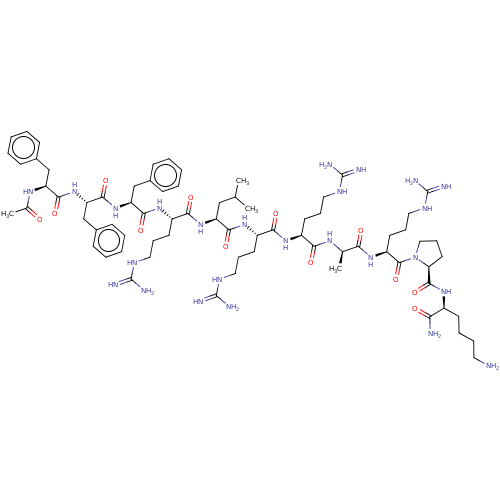 (CHEMBL4290635) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor (unknown origin) | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50027038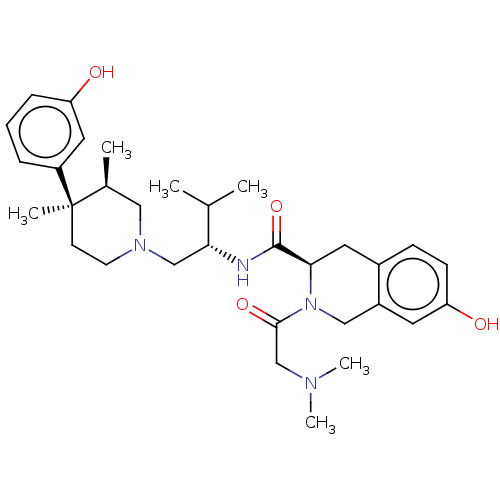 (CHEMBL2112474) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50464460 (CHEMBL4295159) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor (unknown origin) | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50026614 (CHEMBL575508) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50012151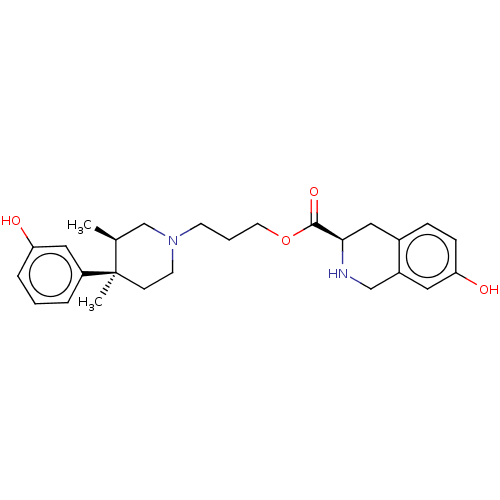 (CHEMBL3264440) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from rat mu-opioid receptor in expressed in rat C6 cell membranes after 1 hr | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50012150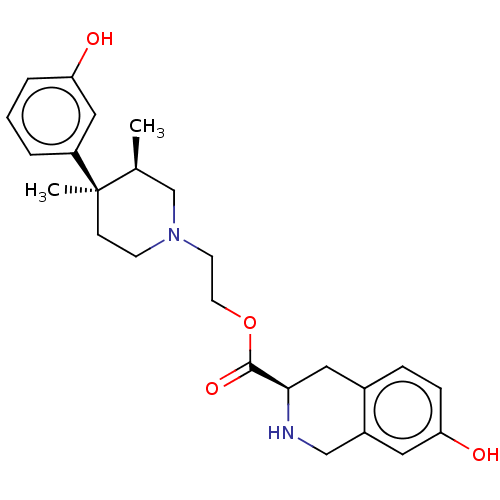 (CHEMBL3264439) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from rat mu-opioid receptor in expressed in rat C6 cell membranes after 1 hr | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012150 (CHEMBL3264439) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50027038 (CHEMBL2112474) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50027051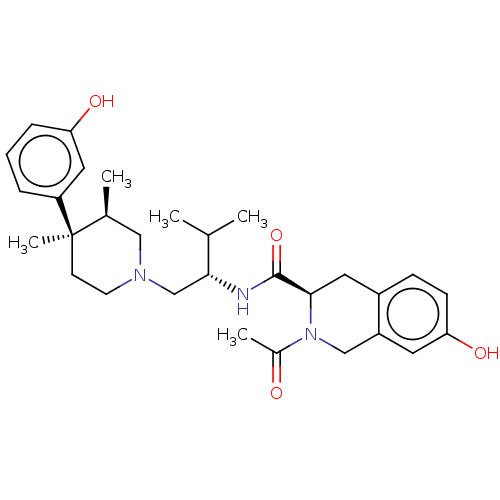 (CHEMBL2112472) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from kappa opioid receptor in guinea pig brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50012151 (CHEMBL3264440) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cells after 1 hr | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50026614 (CHEMBL575508) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 616 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50027051 (CHEMBL2112472) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 764 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50027038 (CHEMBL2112474) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50027051 (CHEMBL2112472) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta-opioid receptor in rat brain membranes after 2 hrs | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50268462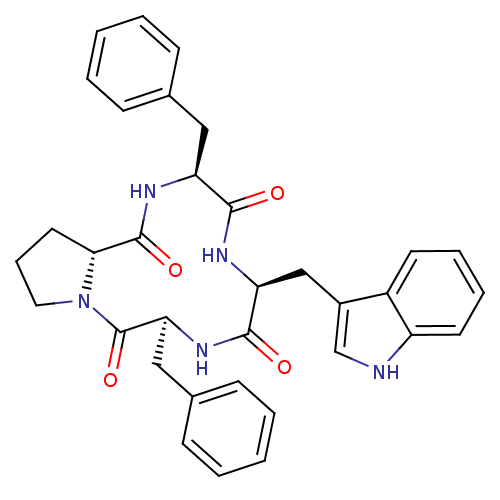 (CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp]) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]CI-977 from kappa opioid receptor in guinea pig brain membranes after 30 mins by scintillation counting method | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50268462 (CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp]) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu-opioid receptor in guinea pig brain membranes after 30 mins by scintillation counting method | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Opioid receptor delta 1 (Cavia porcellus) | BDBM50268462 (CHEMBL506616 | c[L-Phe-D-pro-L-Phe-L-trp]) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from delta-opioid receptor in guinea pig brain membranes after 30 mins by scintillation counting method | Eur J Med Chem 141: 632-647 (2017) Article DOI: 10.1016/j.ejmech.2017.10.012 BindingDB Entry DOI: 10.7270/Q2V98BQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||