

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM111123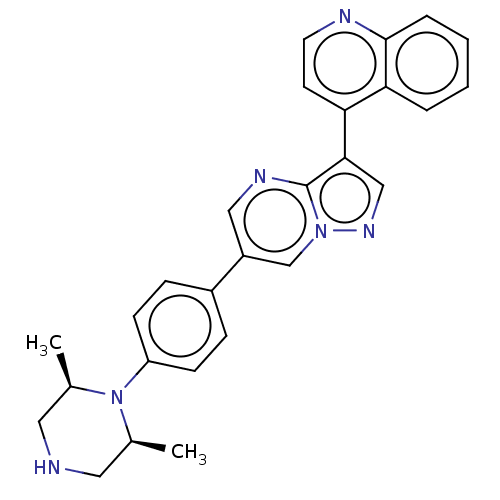 (US10017516, Compound 2 | US9682983, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM111123 (US10017516, Compound 2 | US9682983, 2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466183 (CHEMBL4283638) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466195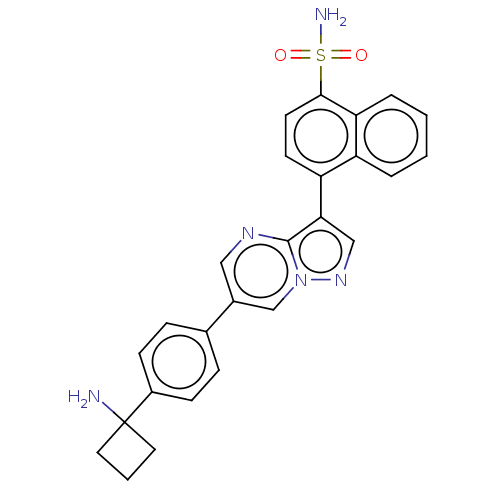 (CHEMBL4278763) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50262079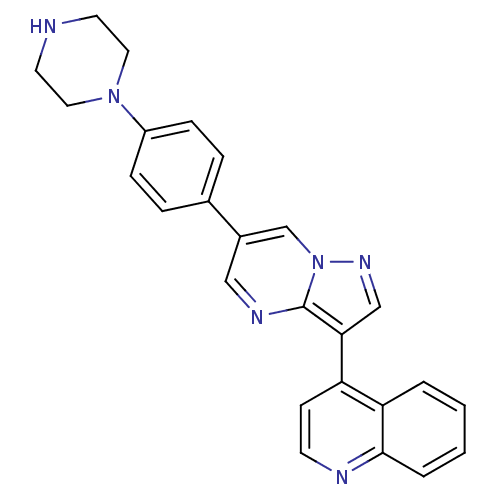 (4-(6-(4-(piperazin-1-yl)phenyl)pyrazolo[1,5-a]pyri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466190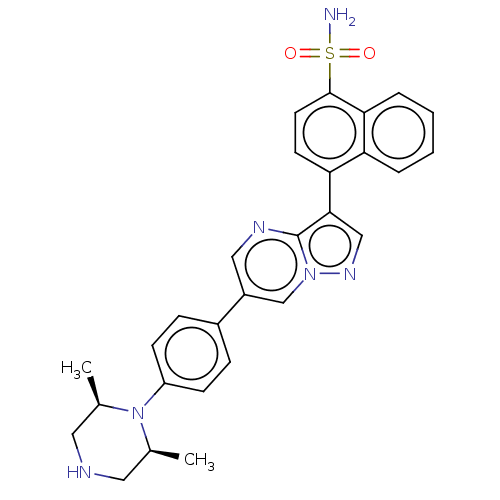 (CHEMBL4288921) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50262079 (4-(6-(4-(piperazin-1-yl)phenyl)pyrazolo[1,5-a]pyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466182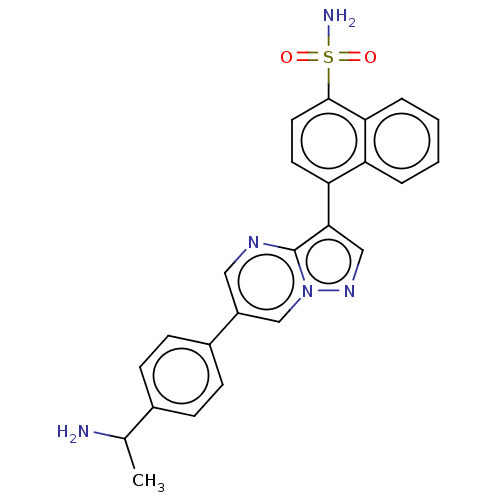 (CHEMBL4293917) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466198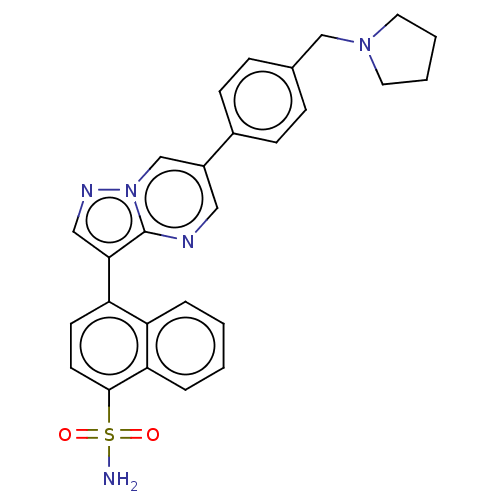 (CHEMBL4281166) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466186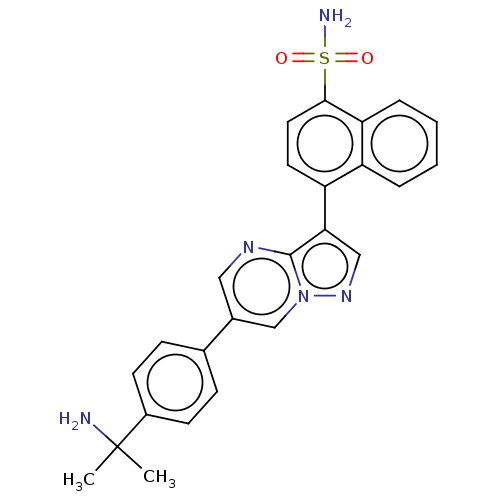 (CHEMBL4283315) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466200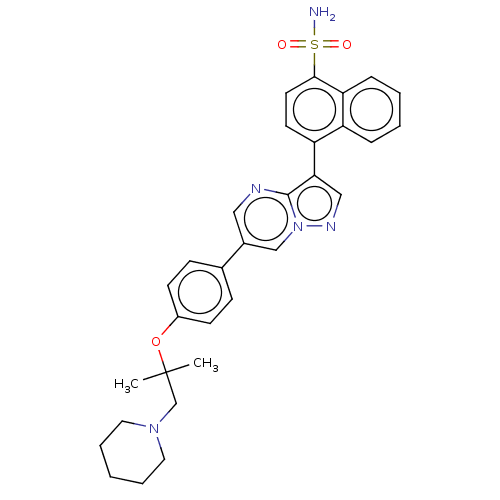 (CHEMBL4278219) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466187 (CHEMBL4288905) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase receptor R3 (Homo sapiens (Human)) | BDBM50466183 (CHEMBL4283638) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK1 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466201 (CHEMBL4283587) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466194 (CHEMBL4285523) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466193 (CHEMBL4277239) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466196 (CHEMBL4281706) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466181 (CHEMBL4285138) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466184 (CHEMBL4292331) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466191 (CHEMBL4278242) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466192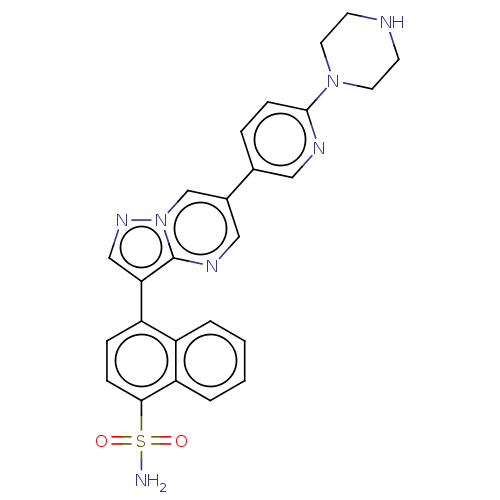 (CHEMBL4280585) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466201 (CHEMBL4283587) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 444 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466189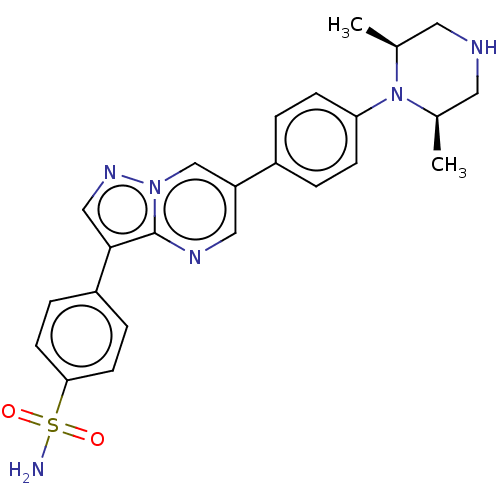 (CHEMBL4277180) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 526 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-2 (Homo sapiens (Human)) | BDBM50466183 (CHEMBL4283638) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 698 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant N-terminal GST-tagged human TGFbetaR2 expressed in Baculovirus expression system by LanthaScreen assay | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466180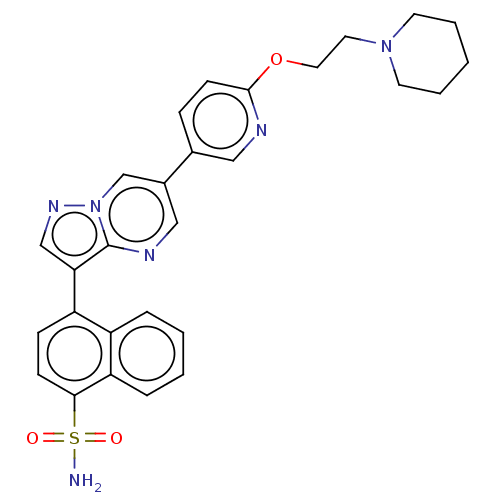 (CHEMBL4290804) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 738 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466197 (CHEMBL4291950) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 796 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466185 (CHEMBL4279135) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466188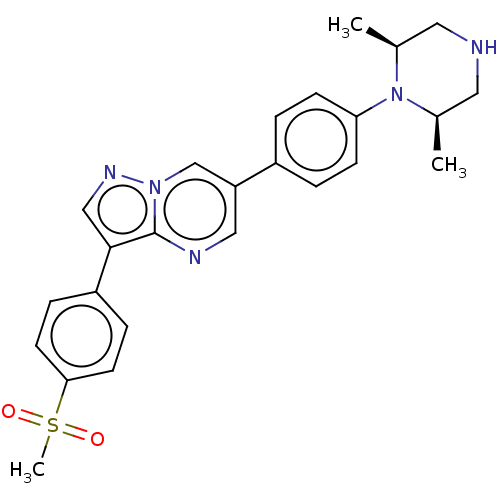 (CHEMBL4288494) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-2A (Homo sapiens (Human)) | BDBM50466183 (CHEMBL4283638) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of full length GST-tagged human ACVR2A expressed in Baculovirus expression system by LanthaScreen assay | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466196 (CHEMBL4281706) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-2B (Homo sapiens (Human)) | BDBM50466183 (CHEMBL4283638) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of GST-tagged human ACVR2B catalytic domain (185 to 488 residues) expressed in Baculovirus expression system by LanthaScreen assay | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466190 (CHEMBL4288921) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50466199 (CHEMBL4291891) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK2 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466183 (CHEMBL4283638) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466195 (CHEMBL4278763) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466197 (CHEMBL4291950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466191 (CHEMBL4278242) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-2 (Homo sapiens (Human)) | BDBM50466183 (CHEMBL4283638) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human BMPR2 (174 to end residues) by LanthaScreen assay | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466194 (CHEMBL4285523) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466193 (CHEMBL4277239) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466185 (CHEMBL4279135) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466192 (CHEMBL4280585) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466184 (CHEMBL4292331) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466187 (CHEMBL4288905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466188 (CHEMBL4288494) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466200 (CHEMBL4278219) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466180 (CHEMBL4290804) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466186 (CHEMBL4283315) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466198 (CHEMBL4281166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein receptor type-1A (Homo sapiens (Human)) | BDBM50466182 (CHEMBL4293917) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of ALK3 (unknown origin) using Ulight topo IIa (Thr 1342) peptide as substrate preincubated for 10 mins followed by substrate addition and... | Bioorg Med Chem Lett 28: 3356-3362 (2018) Article DOI: 10.1016/j.bmcl.2018.09.006 BindingDB Entry DOI: 10.7270/Q2NZ8BBT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |