

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485121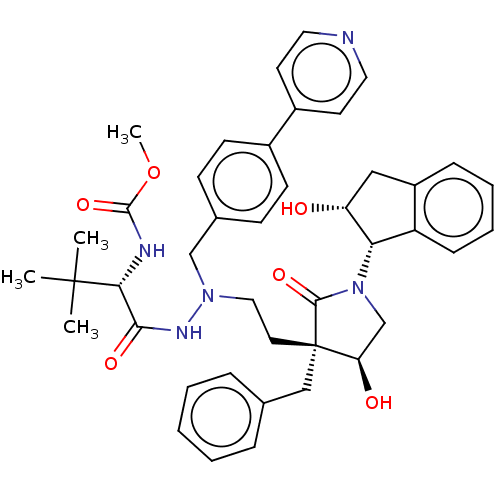 (CHEMBL2030954) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485126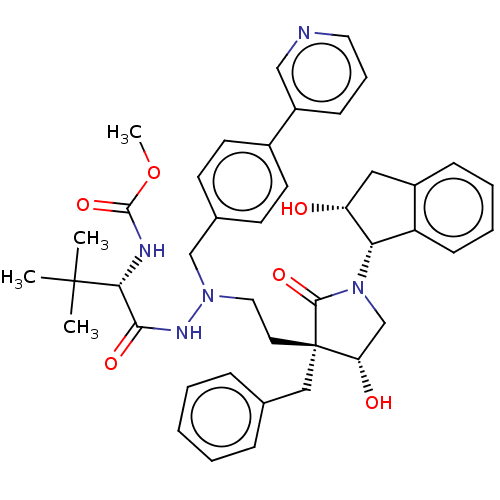 (CHEMBL2030953) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485130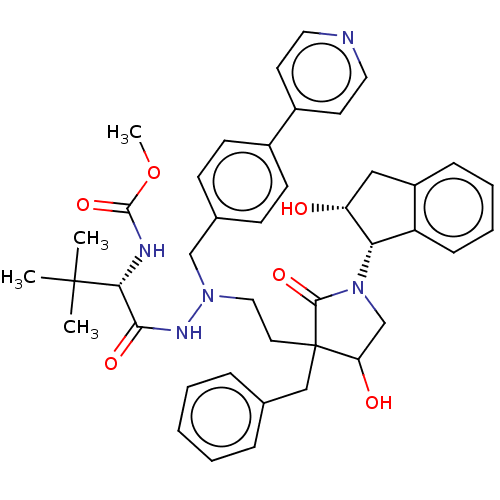 (CHEMBL2031103) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485120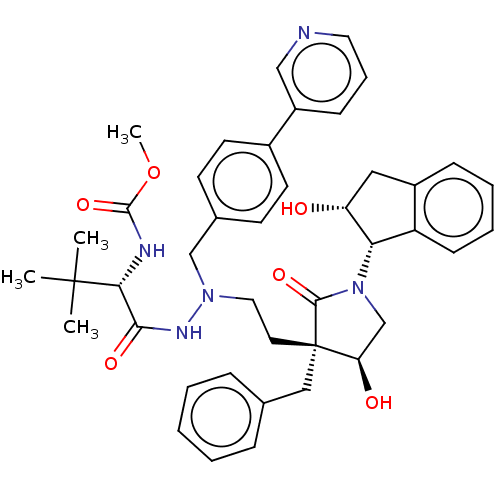 (CHEMBL2030952) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485118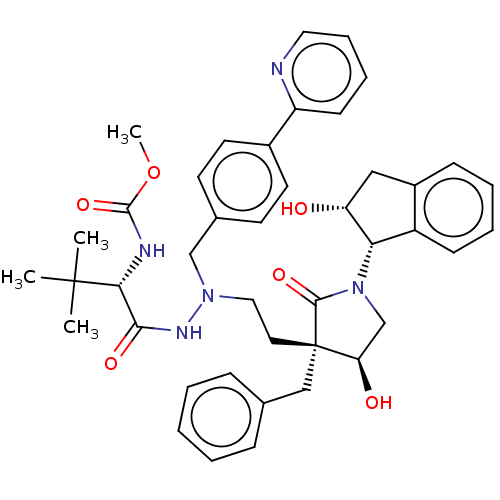 (CHEMBL2030950) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485122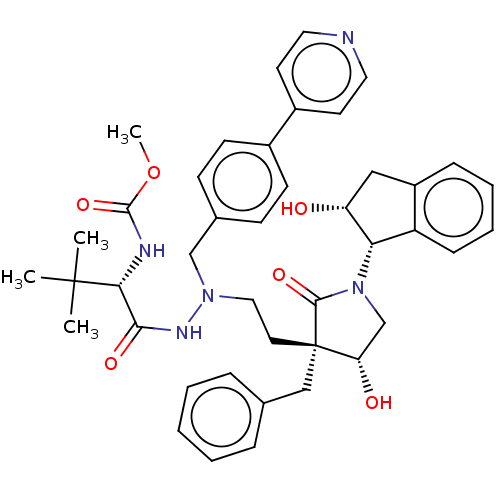 (CHEMBL2030955) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485119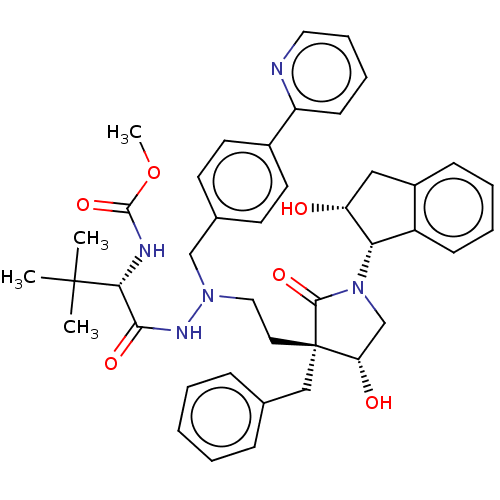 (CHEMBL2030951) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485116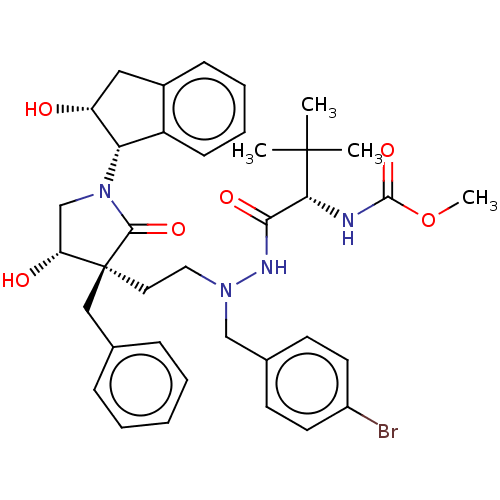 (CHEMBL2030946) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485131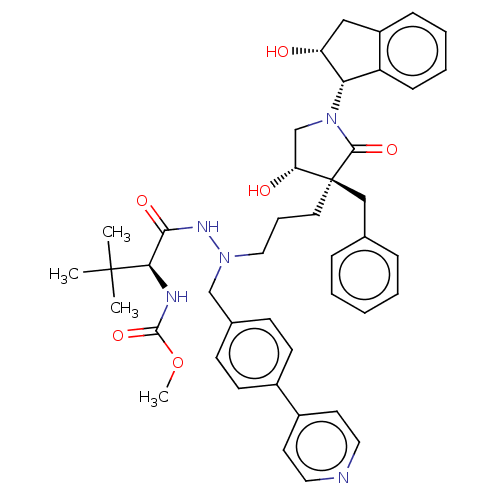 (CHEMBL2031101) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485124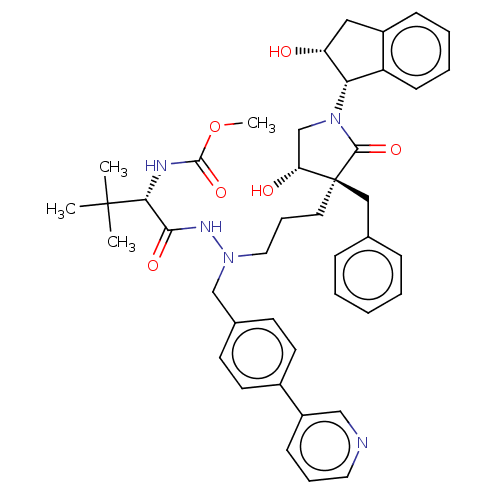 (CHEMBL2031100) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485114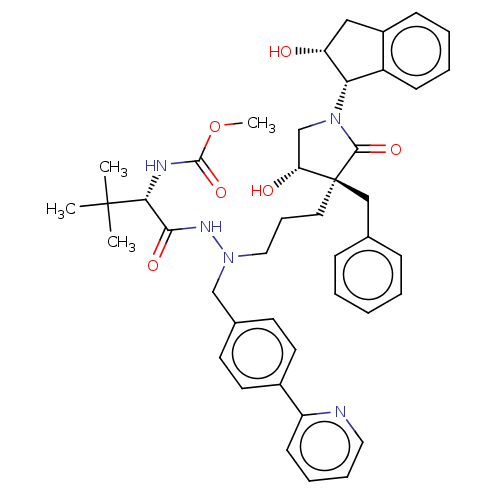 (CHEMBL2031102) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485129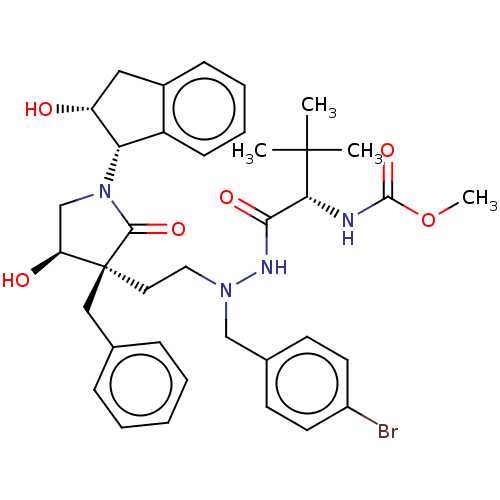 (CHEMBL2030949) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485123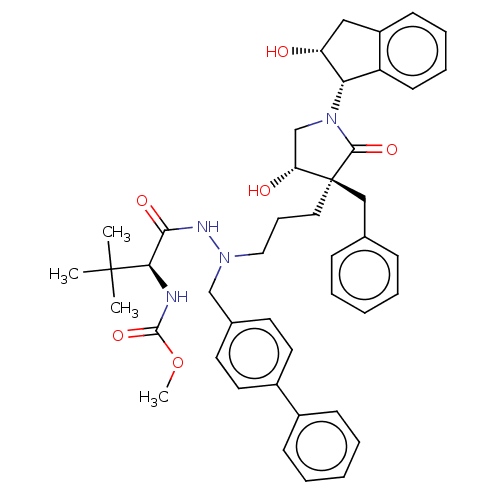 (CHEMBL2031099) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485128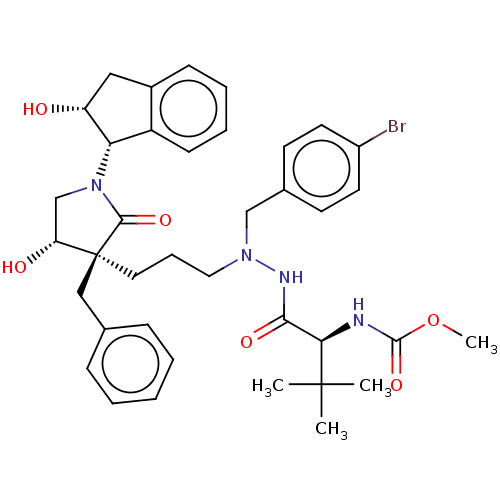 (CHEMBL2030956) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485125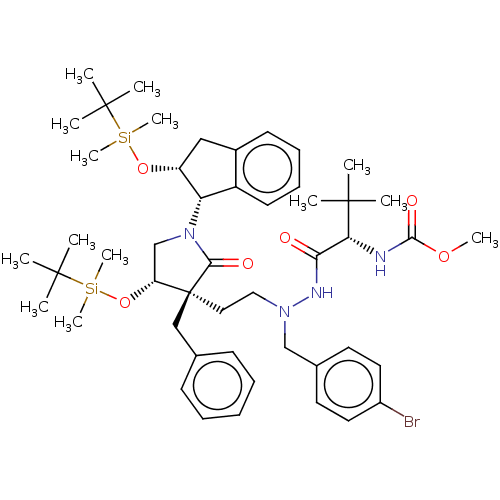 (CHEMBL2030944) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485115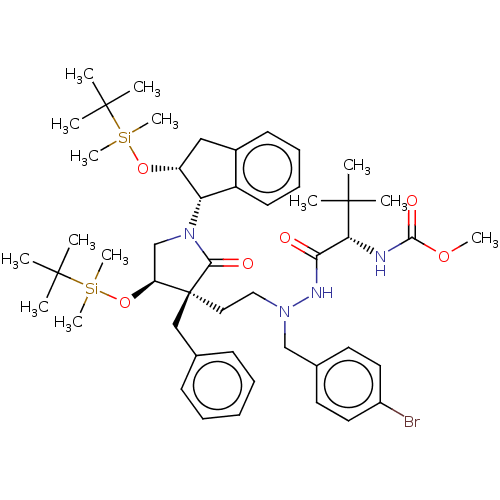 (CHEMBL2030945) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485127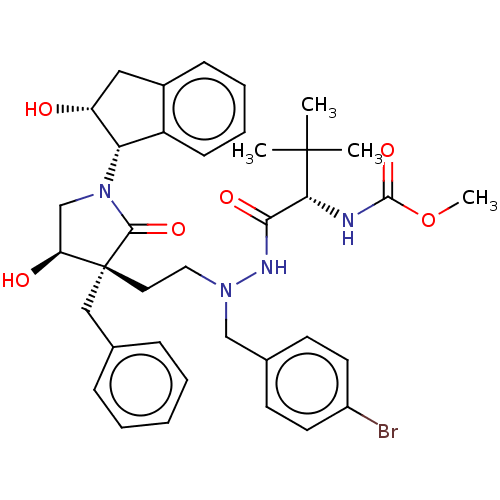 (CHEMBL2030948) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485117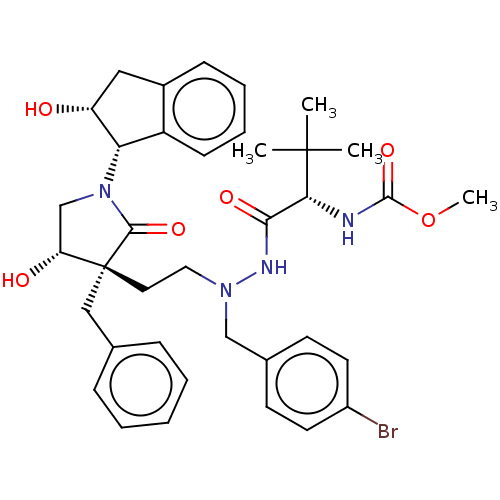 (CHEMBL2030947) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||