

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50031845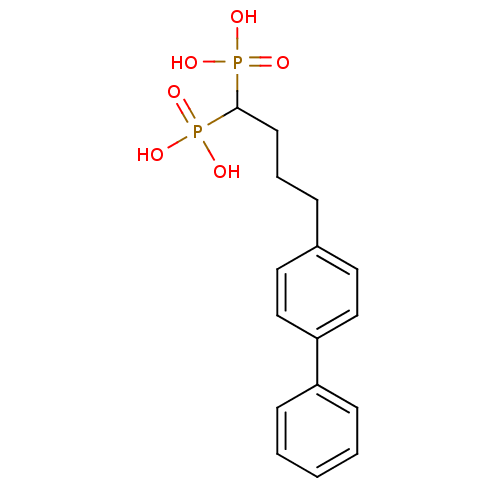 ((4-Biphenyl-4-yl-1-phosphono-butyl)-phosphonic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031839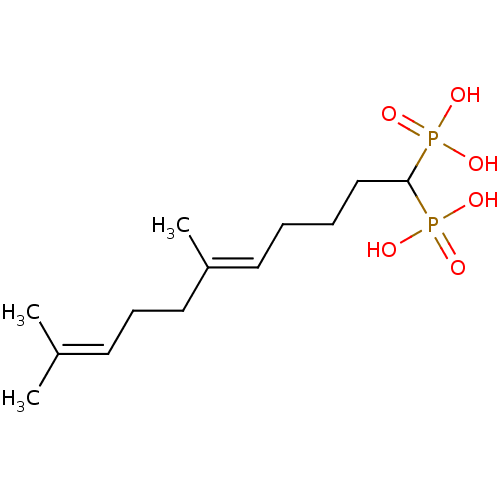 (((E)-6,10-Dimethyl-1-phosphono-undeca-5,9-dienyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031843 (((E)-8,12-Dimethyl-1-phosphono-trideca-7,11-dienyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031848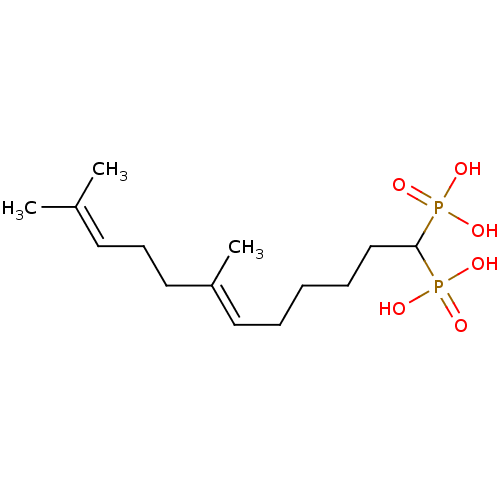 (((E)-7,11-Dimethyl-1-phosphono-dodeca-6,10-dienyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031844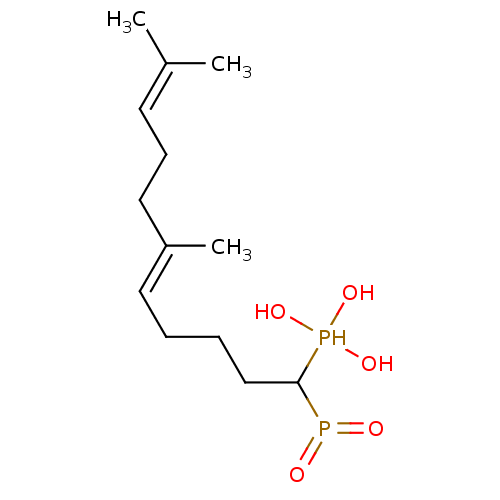 (((E)-1-Hydroxyphosphinoyl-6,10-dimethyl-undeca-5,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031851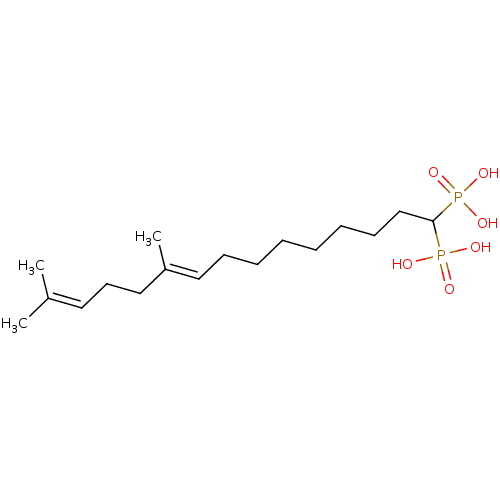 (((E)-10,14-Dimethyl-1-phosphono-pentadeca-9,13-die...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031842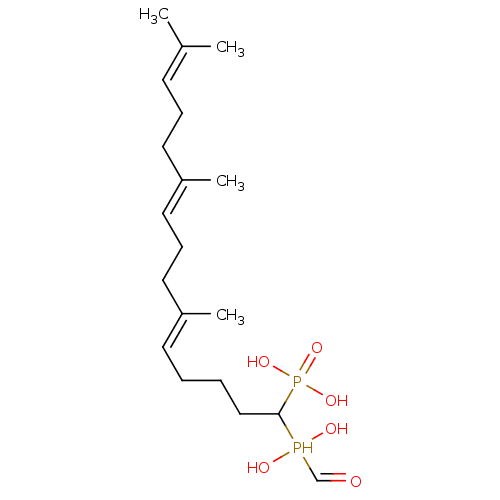 (CHEMBL86867 | [(5E,9E)-1-(Hydroxy-hydroxymethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031847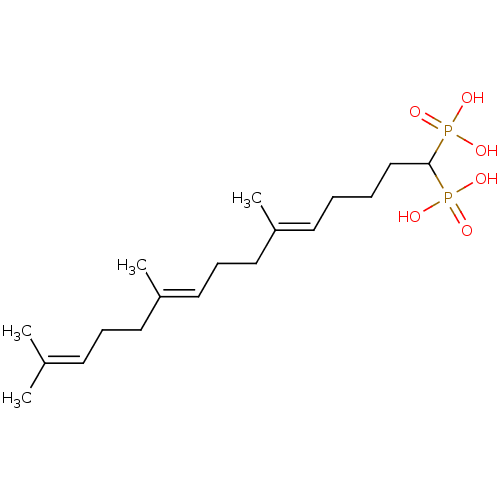 (((5E,9E)-6,10,14-Trimethyl-1-phosphono-pentadeca-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031859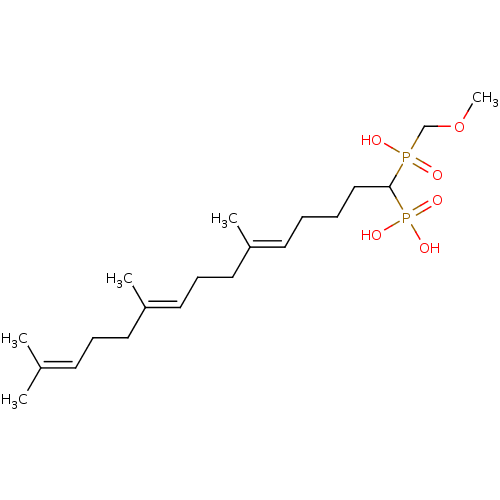 (CHEMBL314797 | [(5E,9E)-1-(Hydroxy-methoxymethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031832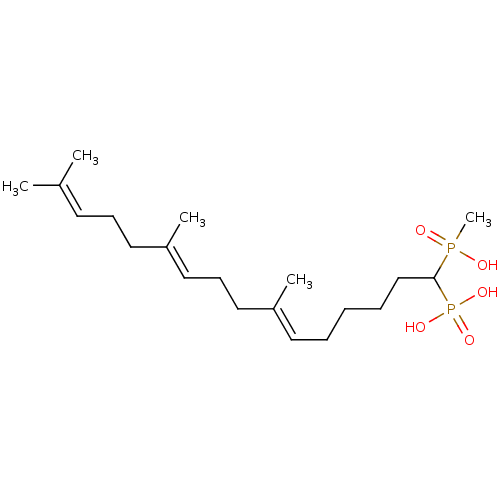 (CHEMBL87922 | [(6E,10E)-1-(Hydroxy-methyl-phosphin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031862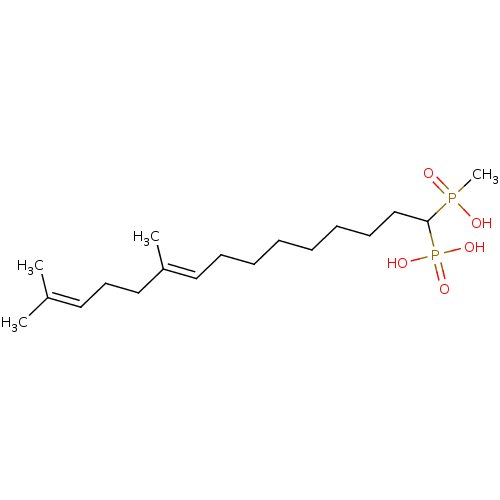 (CHEMBL84629 | [(E)-1-(Hydroxy-methyl-phosphinoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031836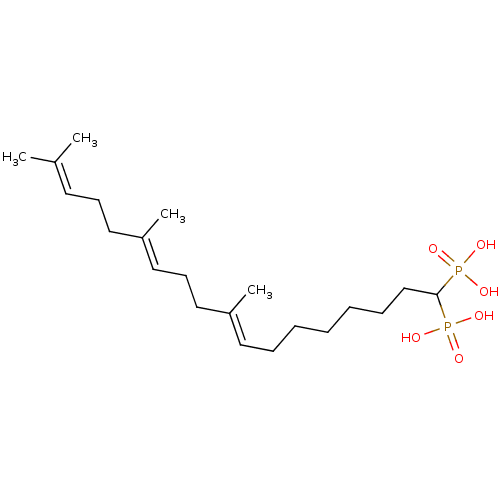 (((8E,12E)-9,13,17-Trimethyl-1-phosphono-octadeca-8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031849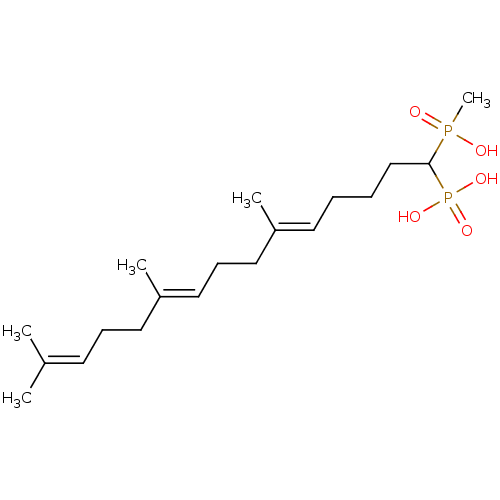 (CHEMBL86918 | [(5E,9E)-1-(Hydroxy-methyl-phosphino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031837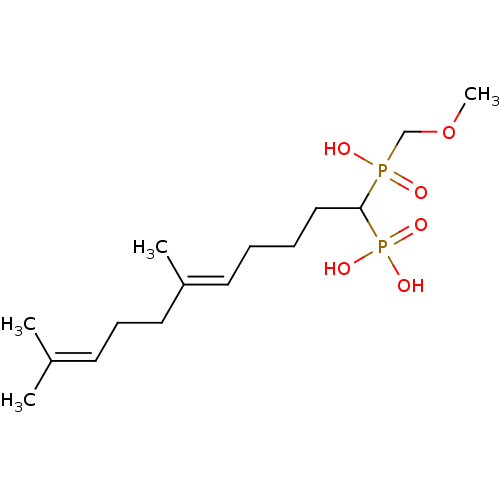 (CHEMBL87489 | [(E)-1-(Hydroxy-methoxymethyl-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031840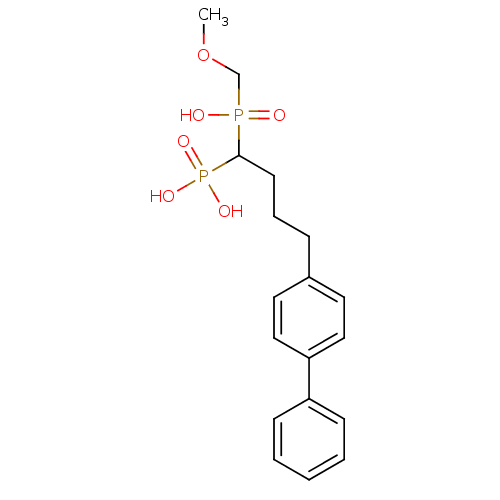 (CHEMBL84961 | [4-Biphenyl-4-yl-1-(hydroxy-methoxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031854 (((E)-9,13-Dimethyl-1-phosphono-tetradeca-8,12-dien...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031855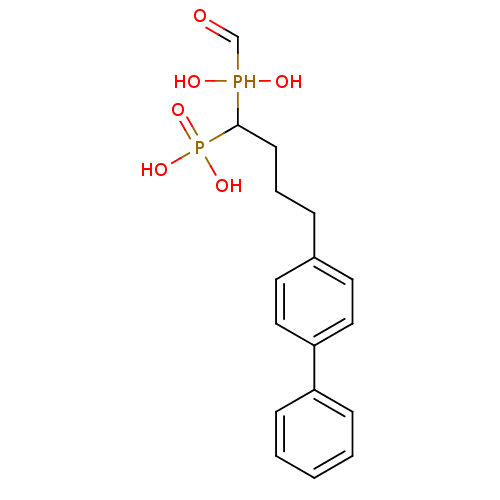 (CHEMBL86621 | [4-Biphenyl-4-yl-1-(hydroxy-hydroxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031850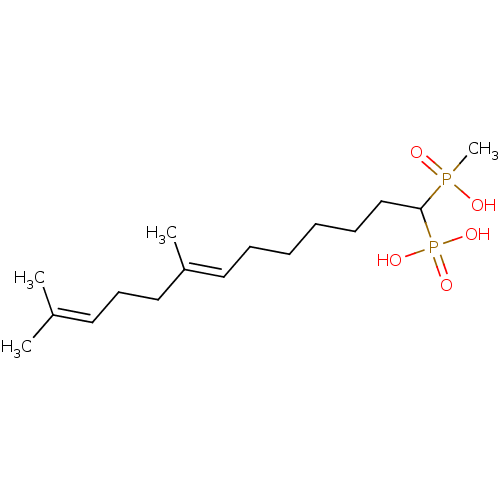 (CHEMBL420416 | [(E)-1-(Hydroxy-methyl-phosphinoyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031853 (((3E,7E)-4,8,12-Trimethyl-1-phosphono-trideca-3,7,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031846 (((E)-4,8-Dimethyl-1-phosphono-nona-3,7-dienyl)-pho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031861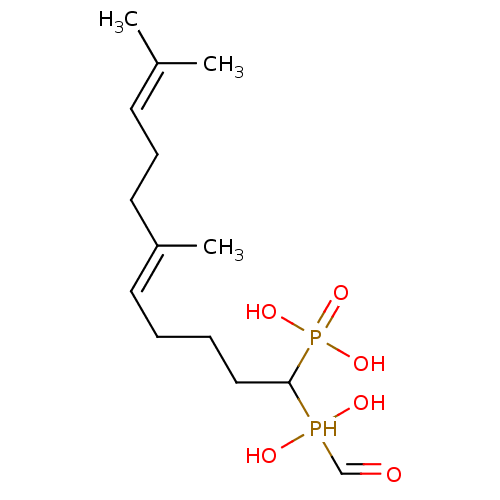 (CHEMBL87822 | [(E)-1-(Hydroxy-hydroxymethyl-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031841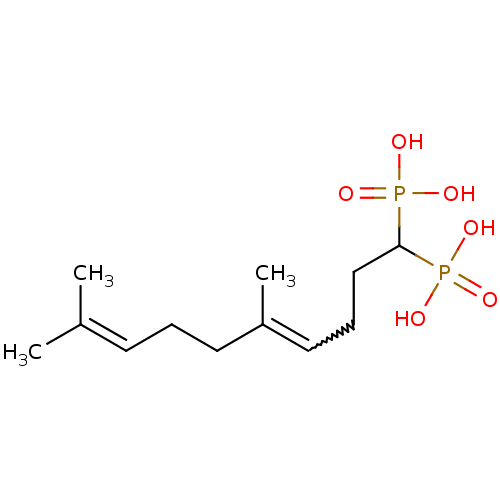 (((E)-5,9-Dimethyl-1-phosphono-deca-4,8-dienyl)-pho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 77.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031858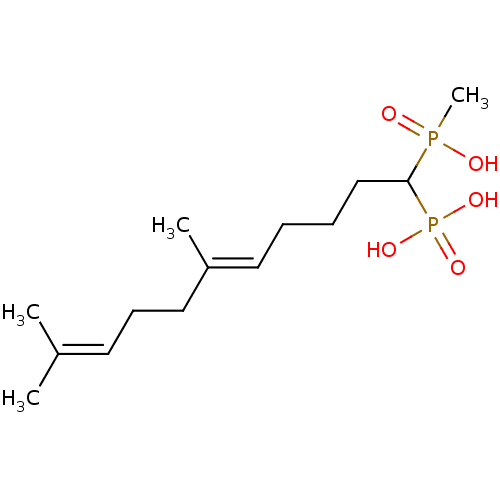 (CHEMBL86580 | [(E)-1-(Hydroxy-methyl-phosphinoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031834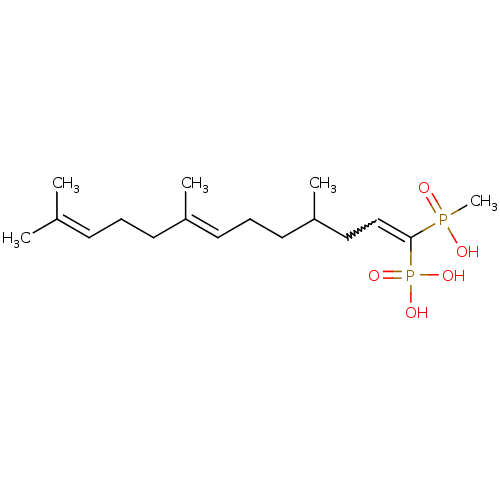 (CHEMBL86919 | [(3E,7E)-1-(Hydroxy-methyl-phosphino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031838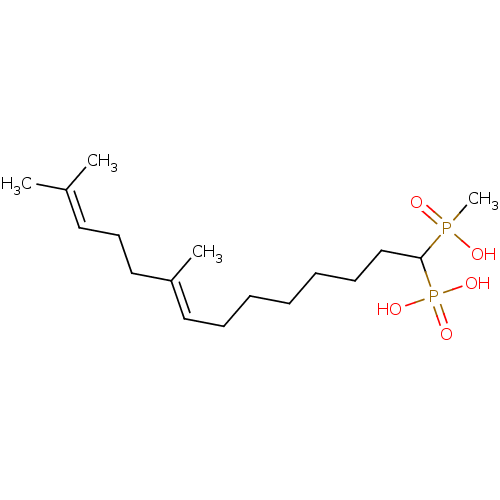 (CHEMBL315239 | [(E)-1-(Hydroxy-methyl-phosphinoyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 374 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031863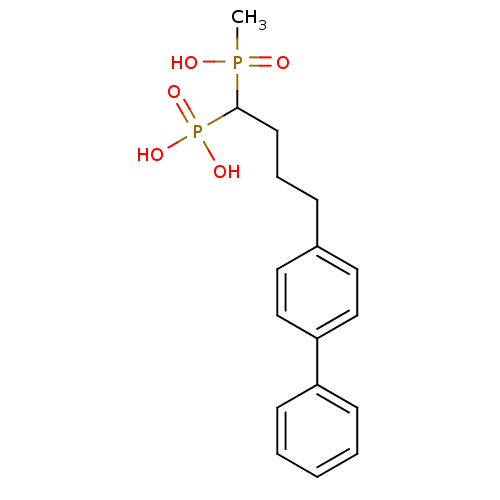 (CHEMBL441807 | [4-Biphenyl-4-yl-1-(hydroxy-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031856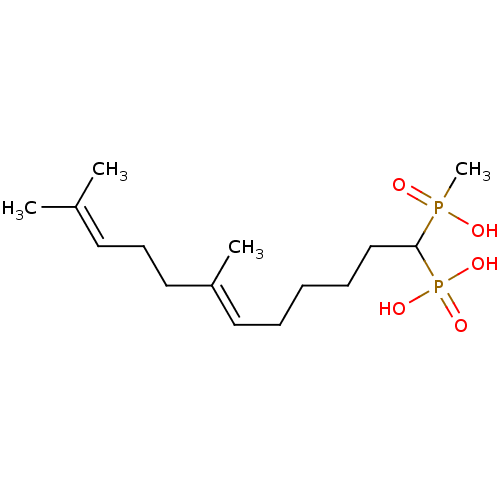 (CHEMBL86707 | [(E)-1-(Hydroxy-methyl-phosphinoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 605 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031835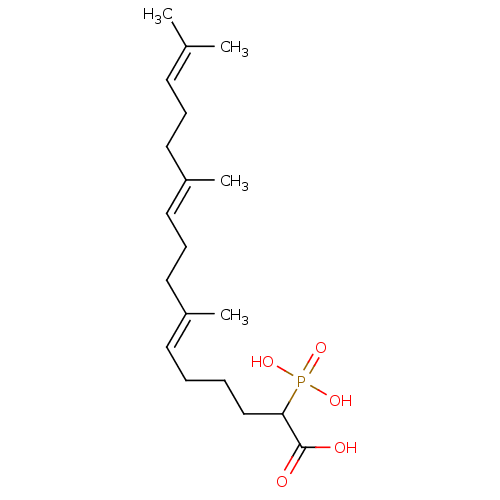 ((6E,10E)-7,11,15-Trimethyl-2-phosphono-hexadeca-6,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031833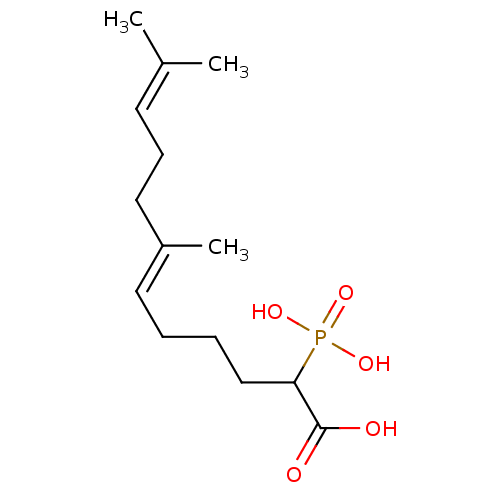 ((E)-7,11-Dimethyl-2-phosphono-dodeca-6,10-dienoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031857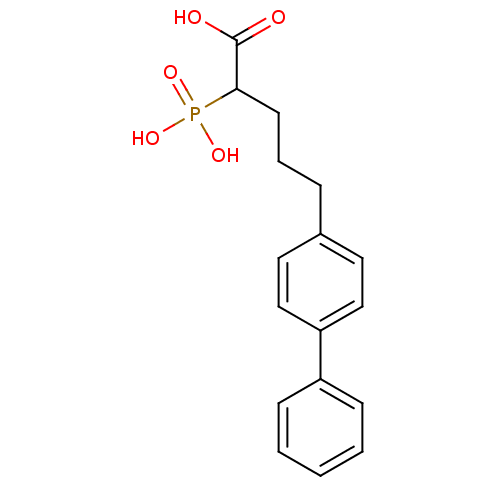 (5-Biphenyl-4-yl-2-phosphono-pentanoic acid | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031852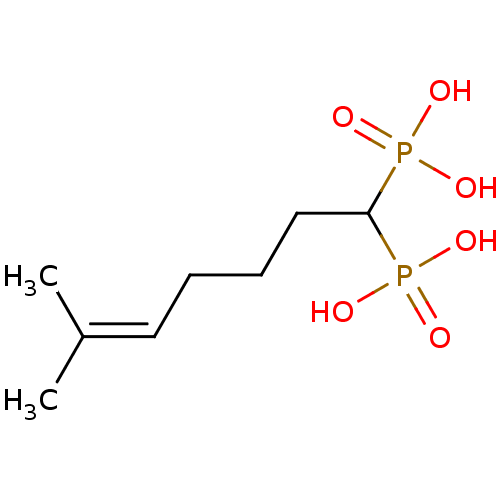 ((6-Methyl-1-phosphono-hept-5-enyl)-phosphonic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031860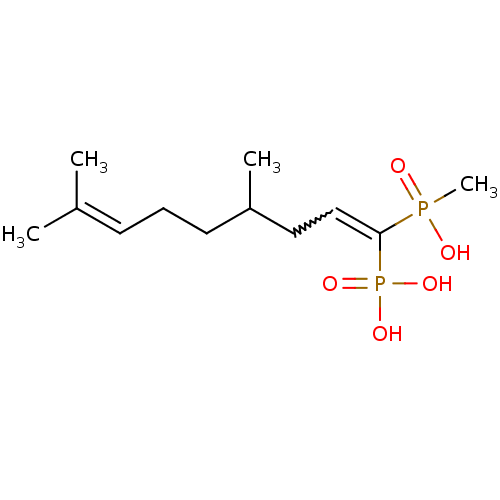 (CHEMBL86632 | [(E)-1-(Hydroxy-methyl-phosphinoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers-Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition rat liver microsomal squalene synthase | J Med Chem 38: 2596-605 (1995) BindingDB Entry DOI: 10.7270/Q2BV7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||