

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493576 (CHEMBL2435828) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493578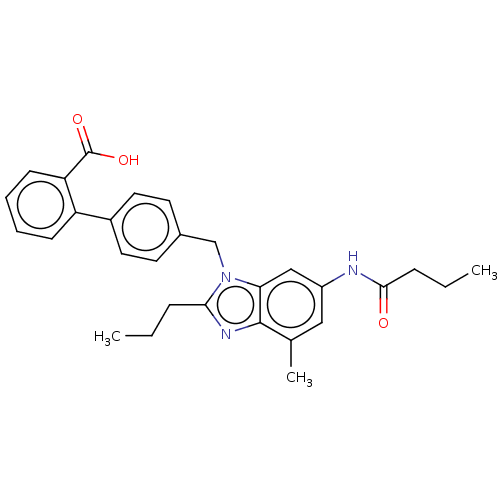 (CHEMBL2435824) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493577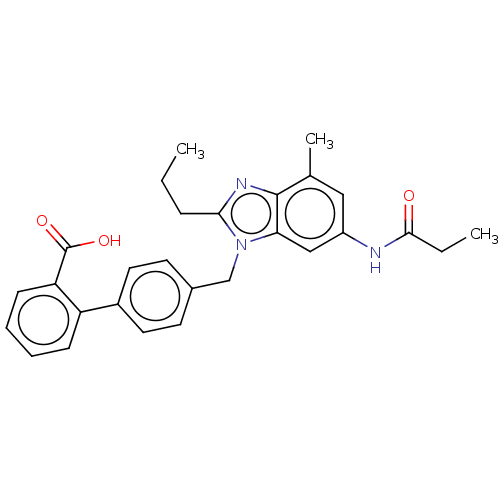 (CHEMBL2435823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493583 (CHEMBL2435829) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50043280 (4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493589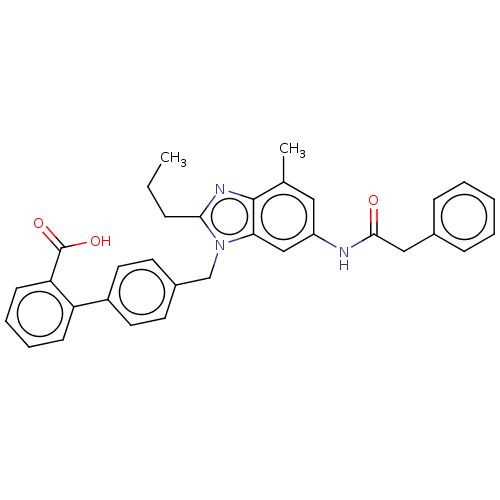 (CHEMBL2435827) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493582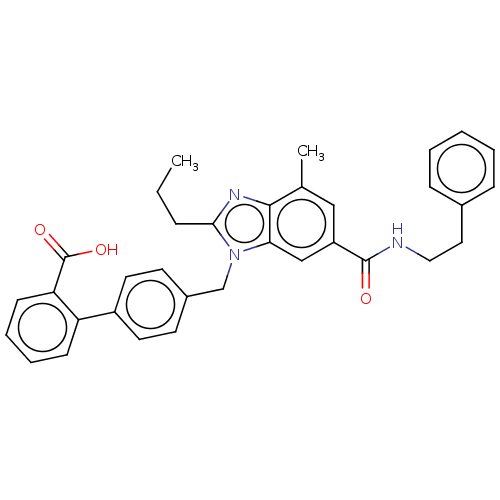 (CHEMBL2435818) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493585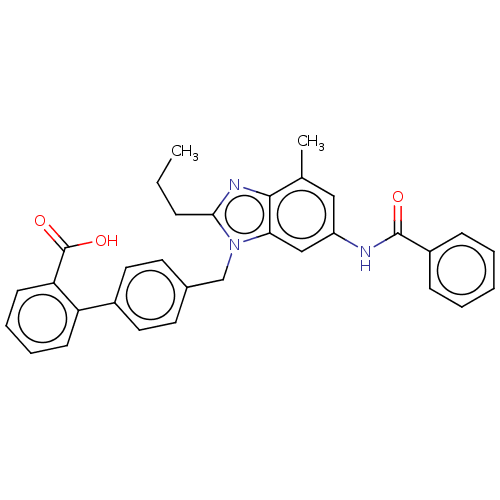 (CHEMBL2435825) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493581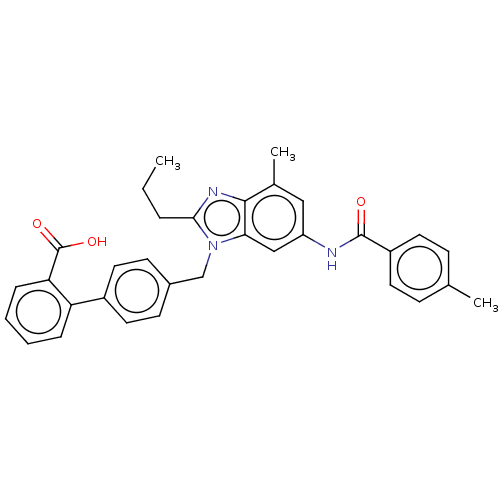 (CHEMBL2435826) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493585 (CHEMBL2435825) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493588 (CHEMBL2435821) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493580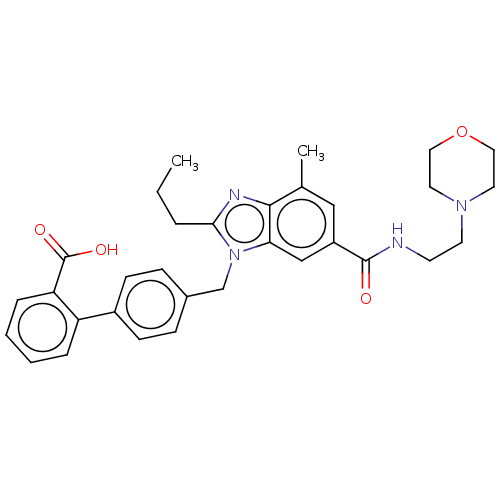 (CHEMBL2435819) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493575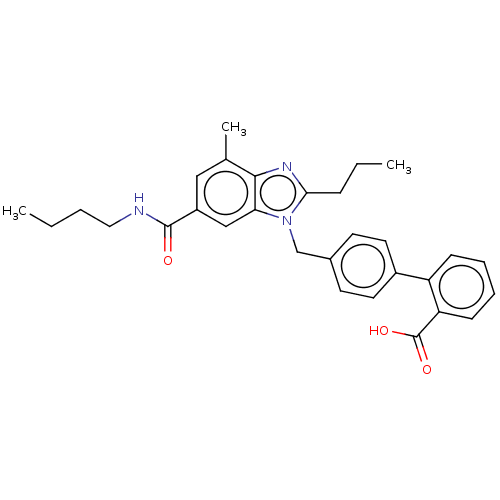 (CHEMBL2435831) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493579 (CHEMBL2435833) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493587 (CHEMBL2435834) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493579 (CHEMBL2435833) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493578 (CHEMBL2435824) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493587 (CHEMBL2435834) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493589 (CHEMBL2435827) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493577 (CHEMBL2435823) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493591 (CHEMBL2435820) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493576 (CHEMBL2435828) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493575 (CHEMBL2435831) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493588 (CHEMBL2435821) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 718 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493586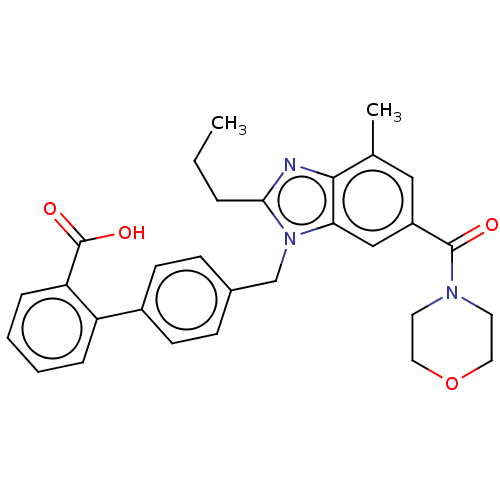 (CHEMBL2435832) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493590 (CHEMBL2435830) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493584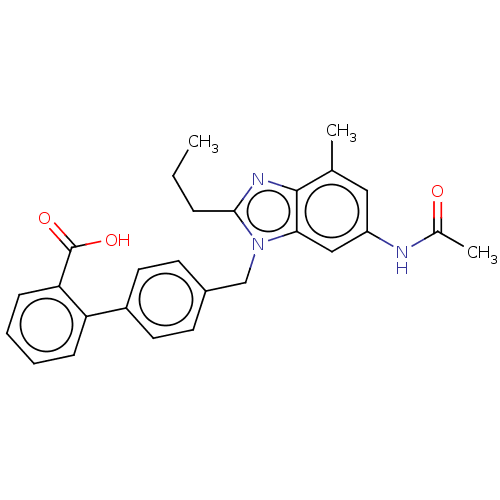 (CHEMBL2435822) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT1 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493583 (CHEMBL2435829) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493580 (CHEMBL2435819) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493582 (CHEMBL2435818) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50493581 (CHEMBL2435826) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1Ile8-angiotensin 2 from angiotensin 2 AT2 receptor (unknown origin) after 180 mins by gamma counting analysis | Eur J Med Chem 69: 44-54 (2013) Article DOI: 10.1016/j.ejmech.2013.08.014 BindingDB Entry DOI: 10.7270/Q2PK0K32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||