 Found 136 hits Enz. Inhib. hit(s) with all data for entry = 50008312
Found 136 hits Enz. Inhib. hit(s) with all data for entry = 50008312 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50517882
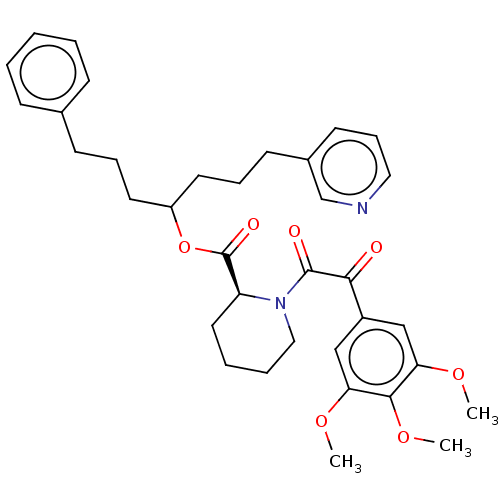
(CHEMBL4449096)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCCC[C@H]1C(=O)OC(CCCc1ccccc1)CCCc1cccnc1 |r| Show InChI InChI=1S/C35H42N2O7/c1-41-30-22-27(23-31(42-2)33(30)43-3)32(38)34(39)37-21-8-7-19-29(37)35(40)44-28(17-9-14-25-12-5-4-6-13-25)18-10-15-26-16-11-20-36-24-26/h4-6,11-13,16,20,22-24,28-29H,7-10,14-15,17-19,21H2,1-3H3/t28?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of His6 tagged human FKBP12 expressed in Escherichia coli BL21(DE3) cells using succinylALPF-p-nitroanilide as substrate by fluorescence p... |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50008984

(4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...)Show InChI InChI=1S/C22H28N2O/c1-2-22(25)24(20-11-7-4-8-12-20)21-14-17-23(18-15-21)16-13-19-9-5-3-6-10-19/h3-12,21H,2,13-18H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) expressed in HEK cells at pH 7.4 |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50502780
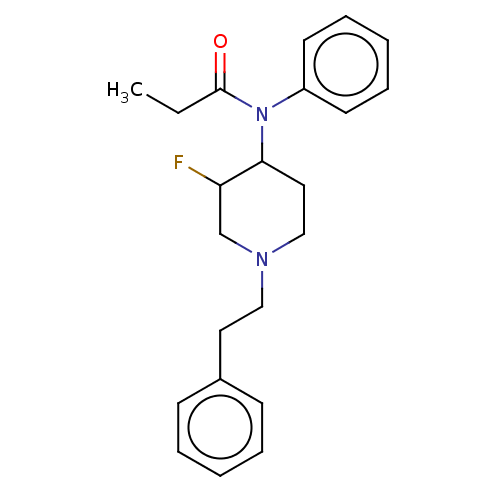
(CHEMBL4471584)Show InChI InChI=1S/C22H27FN2O/c1-2-22(26)25(19-11-7-4-8-12-19)21-14-16-24(17-20(21)23)15-13-18-9-5-3-6-10-18/h3-12,20-21H,2,13-17H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) expressed in HEK cells at pH 6.5 |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to delta opioid receptor (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50502780

(CHEMBL4471584)Show InChI InChI=1S/C22H27FN2O/c1-2-22(26)25(19-11-7-4-8-12-19)21-14-16-24(17-20(21)23)15-13-18-9-5-3-6-10-18/h3-12,20-21H,2,13-17H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) expressed in HEK cells at pH 5.5 |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50517881
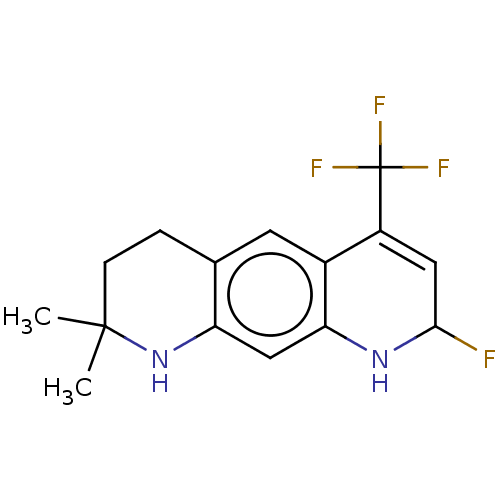
(CHEMBL4548397)Show SMILES CC1(C)CCc2cc3C(=CC(F)Nc3cc2N1)C(F)(F)F |c:8| Show InChI InChI=1S/C15H16F4N2/c1-14(2)4-3-8-5-9-10(15(17,18)19)6-13(16)20-12(9)7-11(8)21-14/h5-7,13,20-21H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human androgen receptor |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50502780

(CHEMBL4471584)Show InChI InChI=1S/C22H27FN2O/c1-2-22(26)25(19-11-7-4-8-12-19)21-14-16-24(17-20(21)23)15-13-18-9-5-3-6-10-18/h3-12,20-21H,2,13-17H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor (unknown origin) expressed in HEK cells at pH 7.4 |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50400862
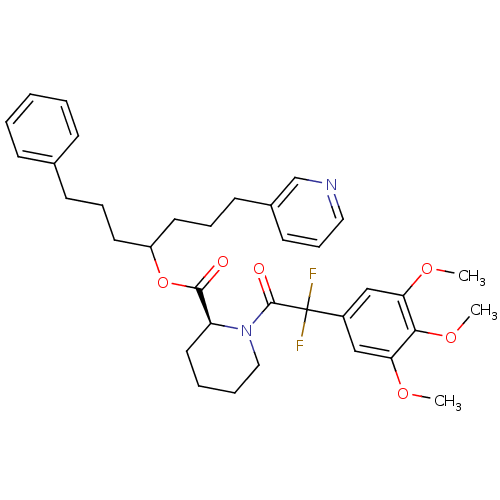
(CHEMBL2205015)Show SMILES COc1cc(cc(OC)c1OC)C(F)(F)C(=O)N1CCCC[C@H]1C(=O)OC(CCCc1ccccc1)CCCc1cccnc1 |r| Show InChI InChI=1S/C35H42F2N2O6/c1-42-30-22-27(23-31(43-2)32(30)44-3)35(36,37)34(41)39-21-8-7-19-29(39)33(40)45-28(17-9-14-25-12-5-4-6-13-25)18-10-15-26-16-11-20-38-24-26/h4-6,11-13,16,20,22-24,28-29H,7-10,14-15,17-19,21H2,1-3H3/t28?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of His6 tagged human FKBP12 expressed in Escherichia coli BL21(DE3) cells using succinylALPF-p-nitroanilide as substrate by fluorescence p... |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50517899

(CHEMBL4447539)Show SMILES CC1(C)CCc2cc3C(=CC(Nc3cc2N1)C#N)C(F)(F)F |c:8| Show InChI InChI=1S/C16H16F3N3/c1-15(2)4-3-9-5-11-12(16(17,18)19)6-10(8-20)21-14(11)7-13(9)22-15/h5-7,10,21-22H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human androgen receptor |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50063127
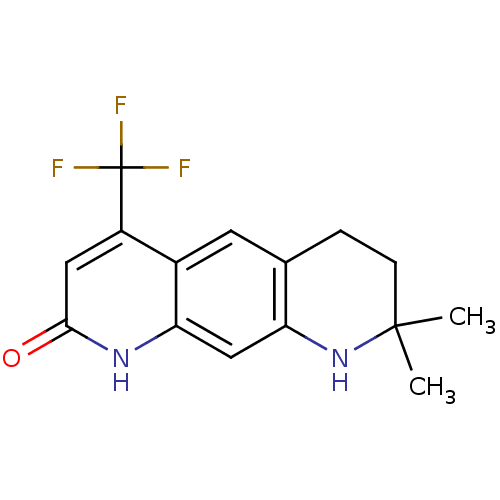
(8,8-Dimethyl-4-trifluoromethyl-6,7,8,9-tetrahydro-...)Show InChI InChI=1S/C15H15F3N2O/c1-14(2)4-3-8-5-9-10(15(16,17)18)6-13(21)19-12(9)7-11(8)20-14/h5-7,20H,3-4H2,1-2H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human androgen receptor |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (isolate Con1) (HCV)) | BDBM50110125

(2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(O)=O Show InChI InChI=1S/C44H56F2N6O14/c1-24(53)47-31(23-36(58)59)42(63)48-29(18-20-35(56)57)40(61)52-38(37(26-13-7-3-8-14-26)27-15-9-4-10-16-27)43(64)49-28(17-19-34(54)55)39(60)50-30(21-25-11-5-2-6-12-25)41(62)51-32(44(65)66)22-33(45)46/h3-4,7-10,13-16,25,28-33,37-38H,2,5-6,11-12,17-23H2,1H3,(H,47,53)(H,48,63)(H,49,64)(H,50,60)(H,51,62)(H,52,61)(H,54,55)(H,56,57)(H,58,59)(H,65,66)/t28-,29-,30-,31-,32-,38-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50517920

(CHEMBL4572725)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)OC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C28H36N4O8/c1-17(2)12-22(28(38)39)32-27(37)23(14-18-6-4-3-5-7-18)40-25(35)16-30-24(34)15-31-26(36)21(29)13-19-8-10-20(33)11-9-19/h3-11,17,21-23,33H,12-16,29H2,1-2H3,(H,30,34)(H,31,36)(H,32,37)(H,38,39)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to delta opioid receptor (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (isolate Con1) (HCV)) | BDBM50084685

(4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C43H56N6O14S/c1-24(50)44-31(22-35(55)56)41(60)45-29(18-20-34(53)54)39(58)49-37(36(26-13-7-3-8-14-26)27-15-9-4-10-16-27)42(61)46-28(17-19-33(51)52)38(57)47-30(21-25-11-5-2-6-12-25)40(59)48-32(23-64)43(62)63/h3-4,7-10,13-16,25,28-32,36-37,64H,2,5-6,11-12,17-23H2,1H3,(H,44,50)(H,45,60)(H,46,61)(H,47,57)(H,48,59)(H,49,58)(H,51,52)(H,53,54)(H,55,56)(H,62,63)/t28-,29-,30-,31-,32-,37-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50517888

(CHEMBL4442842)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)\C=C(/F)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C29H37FN4O6/c1-18(2)12-25(29(39)40)34-27(37)21(13-19-6-4-3-5-7-19)15-22(30)16-32-26(36)17-33-28(38)24(31)14-20-8-10-23(35)11-9-20/h3-11,15,18,21,24-25,35H,12-14,16-17,31H2,1-2H3,(H,32,36)(H,33,38)(H,34,37)(H,39,40)/b22-15-/t21-,24+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to delta opioid receptor (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50517889

(CHEMBL4567560)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=S)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C28H37N5O6S/c1-17(2)12-23(28(38)39)33-27(37)22(14-18-6-4-3-5-7-18)32-25(40)16-30-24(35)15-31-26(36)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,36)(H,32,40)(H,33,37)(H,38,39)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to delta opioid receptor (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50517912
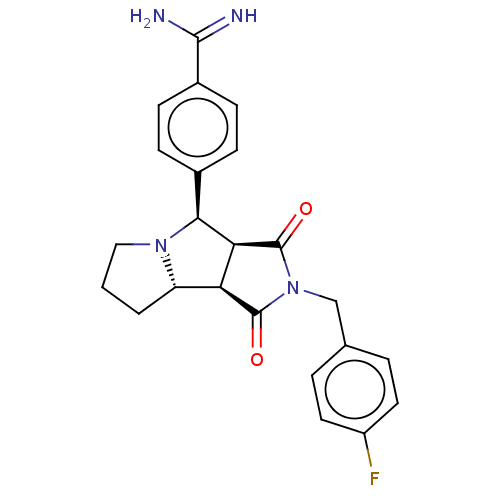
(CHEMBL4586690)Show SMILES Cl.[H][C@@]12CCCN1[C@@H](c1ccc(cc1)C(N)=N)[C@@]1([H])C(=O)N(Cc3ccc(F)cc3)C(=O)[C@@]21[H] |r| Show InChI InChI=1S/C23H23FN4O2.ClH/c24-16-9-3-13(4-10-16)12-28-22(29)18-17-2-1-11-27(17)20(19(18)23(28)30)14-5-7-15(8-6-14)21(25)26;/h3-10,17-20H,1-2,11-12H2,(H3,25,26);1H/t17-,18-,19-,20-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517913
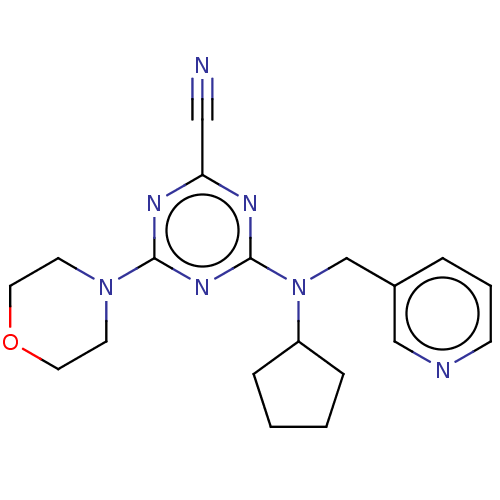
(CHEMBL4449060)Show InChI InChI=1S/C19H23N7O/c20-12-17-22-18(25-8-10-27-11-9-25)24-19(23-17)26(16-5-1-2-6-16)14-15-4-3-7-21-13-15/h3-4,7,13,16H,1-2,5-6,8-11,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517897
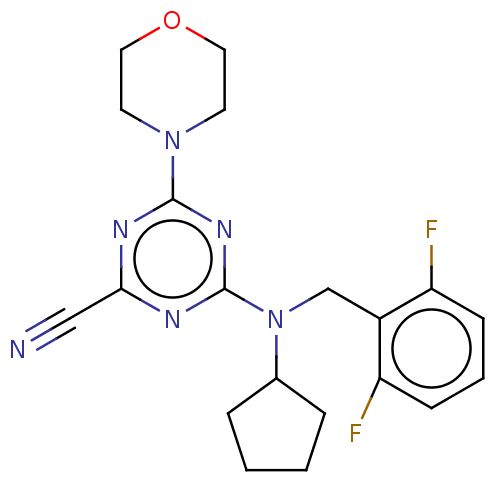
(CHEMBL4520591)Show SMILES Fc1cccc(F)c1CN(C1CCCC1)c1nc(nc(n1)N1CCOCC1)C#N Show InChI InChI=1S/C20H22F2N6O/c21-16-6-3-7-17(22)15(16)13-28(14-4-1-2-5-14)20-25-18(12-23)24-19(26-20)27-8-10-29-11-9-27/h3,6-7,14H,1-2,4-5,8-11,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517914

(CHEMBL4447613)Show SMILES Fc1ccc(CN(C2CCCC2)c2nc(nc(n2)N2CCOCC2)C#N)c(F)c1 Show InChI InChI=1S/C20H22F2N6O/c21-15-6-5-14(17(22)11-15)13-28(16-3-1-2-4-16)20-25-18(12-23)24-19(26-20)27-7-9-29-10-8-27/h5-6,11,16H,1-4,7-10,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517906
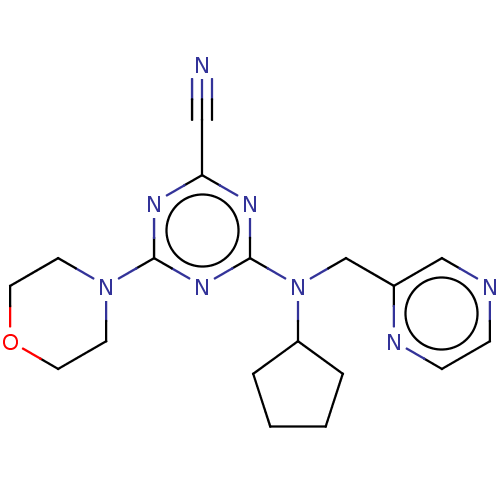
(CHEMBL4450100)Show InChI InChI=1S/C18H22N8O/c19-11-16-22-17(25-7-9-27-10-8-25)24-18(23-16)26(15-3-1-2-4-15)13-14-12-20-5-6-21-14/h5-6,12,15H,1-4,7-10,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517895
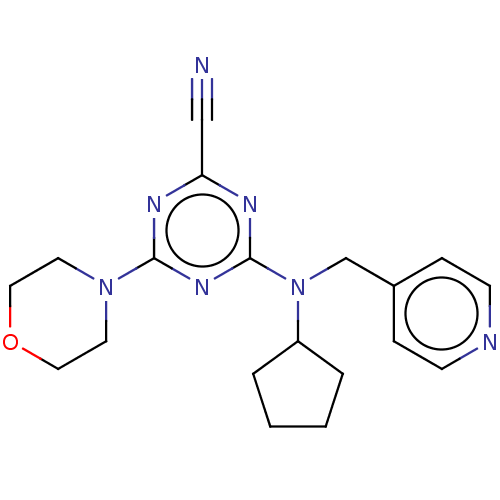
(CHEMBL4458348)Show InChI InChI=1S/C19H23N7O/c20-13-17-22-18(25-9-11-27-12-10-25)24-19(23-17)26(16-3-1-2-4-16)14-15-5-7-21-8-6-15/h5-8,16H,1-4,9-12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50517894

(CHEMBL4529851)Show SMILES Cl.[H][C@@]12CCCN1[C@@H](c1ccc(cc1)C(N)=N)[C@@]1([H])C(=O)N(Cc3ccc(Cl)cc3)C(=O)[C@@]21[H] |r| Show InChI InChI=1S/C23H23ClN4O2.ClH/c24-16-9-3-13(4-10-16)12-28-22(29)18-17-2-1-11-27(17)20(19(18)23(28)30)14-5-7-15(8-6-14)21(25)26;/h3-10,17-20H,1-2,11-12H2,(H3,25,26);1H/t17-,18-,19-,20-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517898

(CHEMBL4547962)Show SMILES Fc1cccc(CN(C2CCCC2)c2nc(nc(n2)N2CCOCC2)C#N)c1 Show InChI InChI=1S/C20H23FN6O/c21-16-5-3-4-15(12-16)14-27(17-6-1-2-7-17)20-24-18(13-22)23-19(25-20)26-8-10-28-11-9-26/h3-5,12,17H,1-2,6-11,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517896
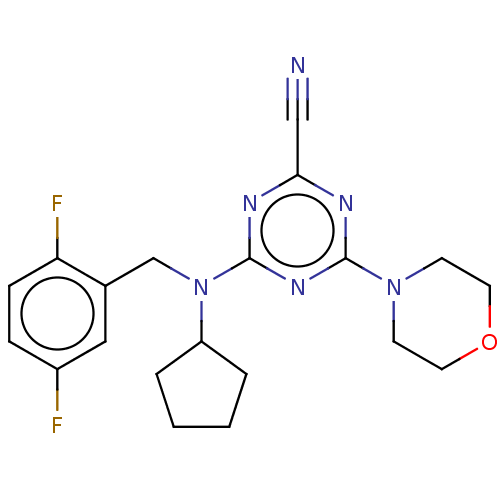
(CHEMBL4542729)Show SMILES Fc1ccc(F)c(CN(C2CCCC2)c2nc(nc(n2)N2CCOCC2)C#N)c1 Show InChI InChI=1S/C20H22F2N6O/c21-15-5-6-17(22)14(11-15)13-28(16-3-1-2-4-16)20-25-18(12-23)24-19(26-20)27-7-9-29-10-8-27/h5-6,11,16H,1-4,7-10,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517917

(CHEMBL4585022)Show InChI InChI=1S/C18H22N8O/c19-11-16-22-17(25-7-9-27-10-8-25)24-18(23-16)26(15-3-1-2-4-15)12-14-5-6-20-13-21-14/h5-6,13,15H,1-4,7-10,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50517891

(CHEMBL4529907)Show SMILES Cl.[H][C@@]12CCCN1[C@@H](c1ccc(cc1)C(N)=N)[C@@]1([H])C(=O)N(Cc3ccc(F)cc3F)C(=O)[C@@]21[H] |r| Show InChI InChI=1S/C23H22F2N4O2.ClH/c24-15-8-7-14(16(25)10-15)11-29-22(30)18-17-2-1-9-28(17)20(19(18)23(29)31)12-3-5-13(6-4-12)21(26)27;/h3-8,10,17-20H,1-2,9,11H2,(H3,26,27);1H/t17-,18-,19-,20-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50517905
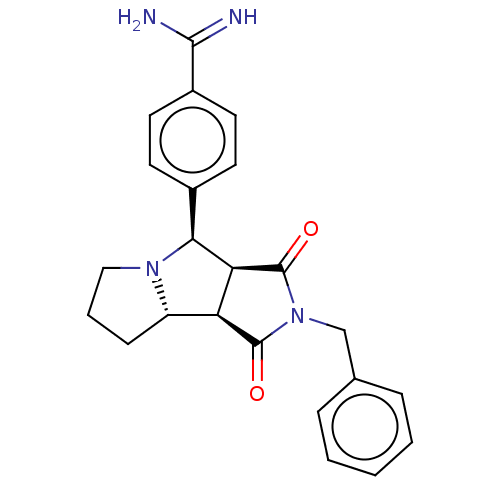
(CHEMBL4441462)Show SMILES Cl.[H][C@@]12CCCN1[C@@H](c1ccc(cc1)C(N)=N)[C@@]1([H])C(=O)N(Cc3ccccc3)C(=O)[C@@]21[H] |r| Show InChI InChI=1S/C23H24N4O2.ClH/c24-21(25)16-10-8-15(9-11-16)20-19-18(17-7-4-12-26(17)20)22(28)27(23(19)29)13-14-5-2-1-3-6-14;/h1-3,5-6,8-11,17-20H,4,7,12-13H2,(H3,24,25);1H/t17-,18-,19-,20-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50517893

(CHEMBL4461448)Show SMILES Cl.[H][C@@]12CCCN1[C@@H](c1ccc(cc1)C(N)=N)[C@@]1([H])C(=O)N(Cc3cccc(F)c3)C(=O)[C@@]21[H] |r| Show InChI InChI=1S/C23H23FN4O2.ClH/c24-16-4-1-3-13(11-16)12-28-22(29)18-17-5-2-10-27(17)20(19(18)23(28)30)14-6-8-15(9-7-14)21(25)26;/h1,3-4,6-9,11,17-20H,2,5,10,12H2,(H3,25,26);1H/t17-,18-,19-,20-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517883

(CHEMBL4440567)Show InChI InChI=1S/C18H22N8O/c19-12-15-22-17(25-8-10-27-11-9-25)24-18(23-15)26(14-4-1-2-5-14)13-16-20-6-3-7-21-16/h3,6-7,14H,1-2,4-5,8-11,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50517892
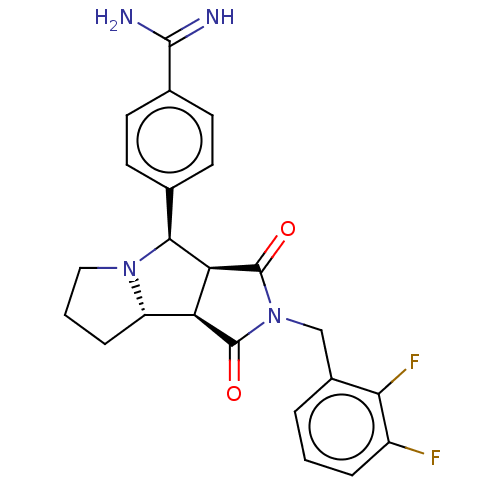
(CHEMBL4513358)Show SMILES Cl.[H][C@@]12CCCN1[C@@H](c1ccc(cc1)C(N)=N)[C@@]1([H])C(=O)N(Cc3cccc(F)c3F)C(=O)[C@@]21[H] |r| Show InChI InChI=1S/C23H22F2N4O2.ClH/c24-15-4-1-3-14(19(15)25)11-29-22(30)17-16-5-2-10-28(16)20(18(17)23(29)31)12-6-8-13(9-7-12)21(26)27;/h1,3-4,6-9,16-18,20H,2,5,10-11H2,(H3,26,27);1H/t16-,17-,18-,20-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50517911

(CHEMBL4574796)Show SMILES Cl.[H][C@@]12CCCN1[C@@H](c1ccc(cc1)C(N)=N)[C@@]1([H])C(=O)N(Cc3ccccc3F)C(=O)[C@@]21[H] |r| Show InChI InChI=1S/C23H23FN4O2.ClH/c24-16-5-2-1-4-15(16)12-28-22(29)18-17-6-3-11-27(17)20(19(18)23(28)30)13-7-9-14(10-8-13)21(25)26;/h1-2,4-5,7-10,17-20H,3,6,11-12H2,(H3,25,26);1H/t17-,18-,19-,20-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50517907

(CHEMBL4463262)Show SMILES Fc1ccc(CN(C2CCCC2)c2nc(nc(n2)N2CCOCC2)C#N)cc1 Show InChI InChI=1S/C20H23FN6O/c21-16-7-5-15(6-8-16)14-27(17-3-1-2-4-17)20-24-18(13-22)23-19(25-20)26-9-11-28-12-10-26/h5-8,17H,1-4,9-12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin L |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50517919
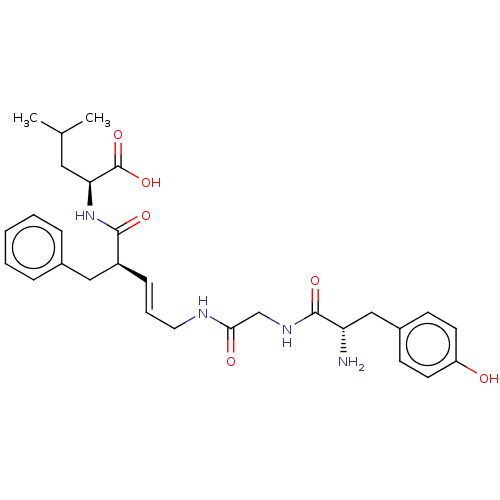
(CHEMBL4445059)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)\C=C\CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C29H38N4O6/c1-19(2)15-25(29(38)39)33-27(36)22(16-20-7-4-3-5-8-20)9-6-14-31-26(35)18-32-28(37)24(30)17-21-10-12-23(34)13-11-21/h3-13,19,22,24-25,34H,14-18,30H2,1-2H3,(H,31,35)(H,32,37)(H,33,36)(H,38,39)/b9-6+/t22-,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 587 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Binding affinity to delta opioid receptor (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus genotype 1b (isolate Con1) (HCV)) | BDBM50110118

(2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC[C@H](NC(=O)[C@H](CC1CCCCC1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(c1ccccc1)c1ccccc1)C(O)=O Show InChI InChI=1S/C44H58N6O14/c1-3-29(44(63)64)46-41(60)32(23-26-13-7-4-8-14-26)49-39(58)30(19-21-34(52)53)48-43(62)38(37(27-15-9-5-10-16-27)28-17-11-6-12-18-28)50-40(59)31(20-22-35(54)55)47-42(61)33(24-36(56)57)45-25(2)51/h5-6,9-12,15-18,26,29-33,37-38H,3-4,7-8,13-14,19-24H2,1-2H3,(H,45,51)(H,46,60)(H,47,61)(H,48,62)(H,49,58)(H,50,59)(H,52,53)(H,54,55)(H,56,57)(H,63,64)/t29-,30-,31-,32-,33-,38-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of HCV NS3 protease |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50517909
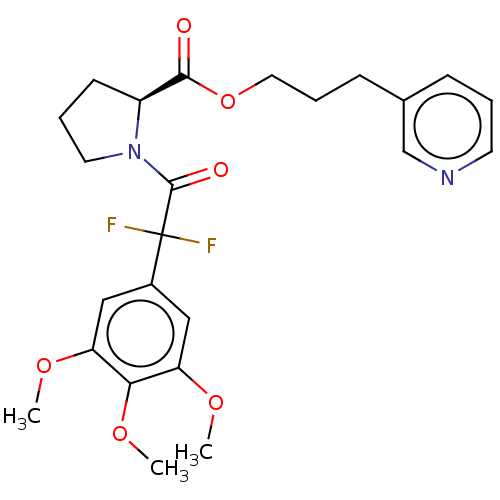
(CHEMBL4458079)Show SMILES COc1cc(cc(OC)c1OC)C(F)(F)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C24H28F2N2O6/c1-31-19-13-17(14-20(32-2)21(19)33-3)24(25,26)23(30)28-11-5-9-18(28)22(29)34-12-6-8-16-7-4-10-27-15-16/h4,7,10,13-15,18H,5-6,8-9,11-12H2,1-3H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of His6 tagged human FKBP12 expressed in Escherichia coli BL21(DE3) cells using succinylALPF-p-nitroanilide as substrate by fluorescence p... |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50517900

(CHEMBL4567340)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1CCC[C@H]1C(=O)OCCCc1cccnc1 |r| Show InChI InChI=1S/C24H28N2O7/c1-30-19-13-17(14-20(31-2)22(19)32-3)21(27)23(28)26-11-5-9-18(26)24(29)33-12-6-8-16-7-4-10-25-15-16/h4,7,10,13-15,18H,5-6,8-9,11-12H2,1-3H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of His6 tagged human FKBP12 expressed in Escherichia coli BL21(DE3) cells using succinylALPF-p-nitroanilide as substrate by fluorescence p... |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50517904
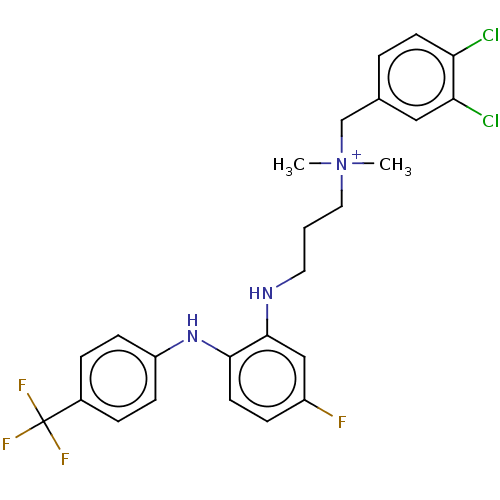
(CHEMBL4461049)Show SMILES C[N+](C)(CCCNc1cc(F)ccc1Nc1ccc(cc1)C(F)(F)F)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C25H26Cl2F4N3/c1-34(2,16-17-4-10-21(26)22(27)14-17)13-3-12-32-24-15-19(28)7-11-23(24)33-20-8-5-18(6-9-20)25(29,30)31/h4-11,14-15,32-33H,3,12-13,16H2,1-2H3/q+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Competitive inhibition of trypanosoma cruzi trypanothione reductase |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50370691
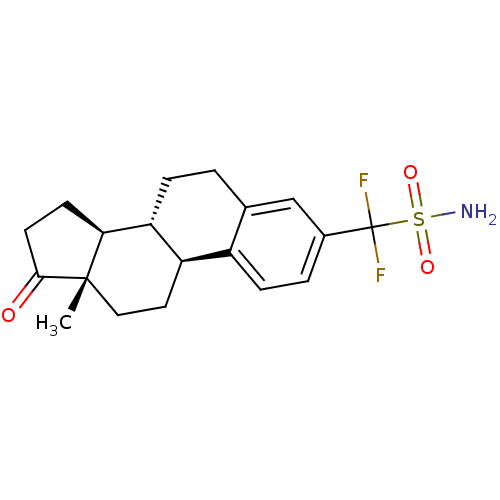
(CHEMBL1628004)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(ccc34)C(F)(F)S(N)(=O)=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H23F2NO3S/c1-18-9-8-14-13-5-3-12(19(20,21)26(22,24)25)10-11(13)2-4-15(14)16(18)6-7-17(18)23/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,22,24,25)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) at pH 8.8 |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50517916
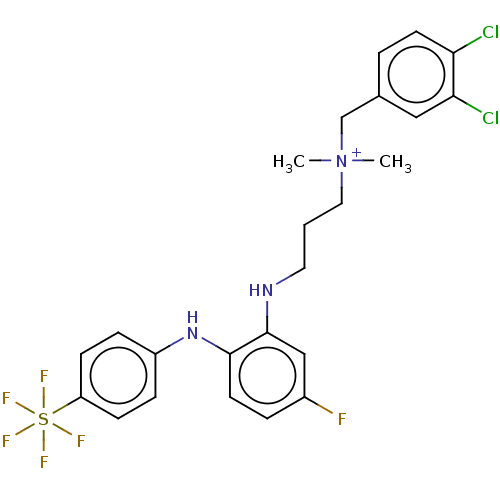
(CHEMBL4450332)Show SMILES C[N+](C)(CCCNc1cc(F)ccc1Nc1ccc(cc1)S(F)(F)(F)(F)F)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C24H26Cl2F6N3S/c1-35(2,16-17-4-10-21(25)22(26)14-17)13-3-12-33-24-15-18(27)5-11-23(24)34-19-6-8-20(9-7-19)36(28,29,30,31)32/h4-11,14-15,33-34H,3,12-13,16H2,1-2H3/q+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Competitive inhibition of trypanosoma cruzi trypanothione reductase |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50366886

(CHEMBL1628110)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(ccc34)C(F)(F)S(O)(=O)=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H22F2O4S/c1-18-9-8-14-13-5-3-12(19(20,21)26(23,24)25)10-11(13)2-4-15(14)16(18)6-7-17(18)22/h3,5,10,14-16H,2,4,6-9H2,1H3,(H,23,24,25)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50366886

(CHEMBL1628110)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(ccc34)C(F)(F)S(O)(=O)=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H22F2O4S/c1-18-9-8-14-13-5-3-12(19(20,21)26(23,24)25)10-11(13)2-4-15(14)16(18)6-7-17(18)22/h3,5,10,14-16H,2,4,6-9H2,1H3,(H,23,24,25)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) at pH 7 |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50370691

(CHEMBL1628004)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(ccc34)C(F)(F)S(N)(=O)=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H23F2NO3S/c1-18-9-8-14-13-5-3-12(19(20,21)26(22,24)25)10-11(13)2-4-15(14)16(18)6-7-17(18)23/h3,5,10,14-16H,2,4,6-9H2,1H3,(H2,22,24,25)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) at pH 7 |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Trypanothione reductase
(Trypanosoma cruzi) | BDBM50517921
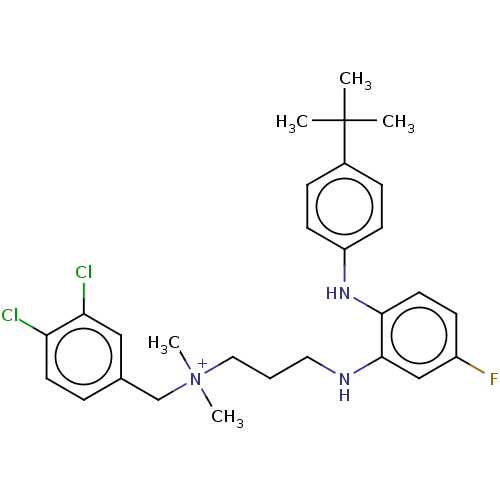
(CHEMBL4562780)Show SMILES CC(C)(C)c1ccc(Nc2ccc(F)cc2NCCC[N+](C)(C)Cc2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C28H35Cl2FN3/c1-28(2,3)21-8-11-23(12-9-21)33-26-14-10-22(31)18-27(26)32-15-6-16-34(4,5)19-20-7-13-24(29)25(30)17-20/h7-14,17-18,32-33H,6,15-16,19H2,1-5H3/q+1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Competitive inhibition of trypanosoma cruzi trypanothione reductase |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50366886

(CHEMBL1628110)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(ccc34)C(F)(F)S(O)(=O)=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H22F2O4S/c1-18-9-8-14-13-5-3-12(19(20,21)26(23,24)25)10-11(13)2-4-15(14)16(18)6-7-17(18)22/h3,5,10,14-16H,2,4,6-9H2,1H3,(H,23,24,25)/t14-,15-,16+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) at pH 8.8 |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50370690
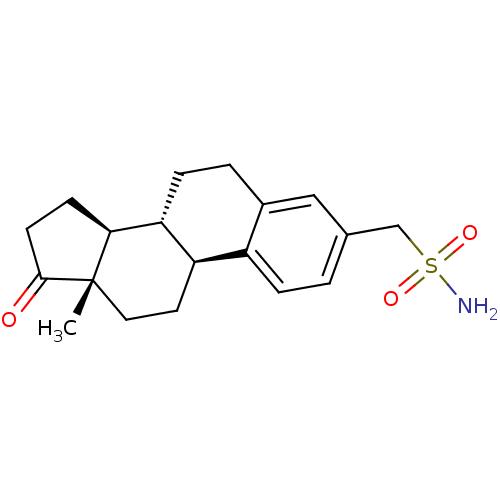
(CHEMBL1628081)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(CS(N)(=O)=O)ccc34)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H25NO3S/c1-19-9-8-15-14-4-2-12(11-24(20,22)23)10-13(14)3-5-16(15)17(19)6-7-18(19)21/h2,4,10,15-17H,3,5-9,11H2,1H3,(H2,20,22,23)/t15-,16-,17+,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) at pH 7 |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50366884
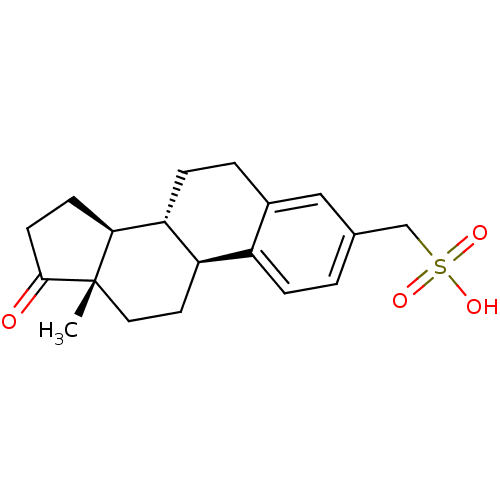
(CHEMBL1628109)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(CS(O)(=O)=O)ccc34)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H24O4S/c1-19-9-8-15-14-4-2-12(11-24(21,22)23)10-13(14)3-5-16(15)17(19)6-7-18(19)20/h2,4,10,15-17H,3,5-9,11H2,1H3,(H,21,22,23)/t15-,16-,17+,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50082127
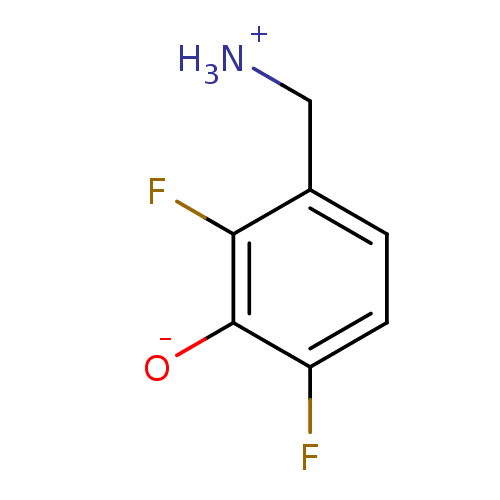
(3-(ammoniomethyl)-2,6-difluorobenzenolate | 3-Amin...)Show InChI InChI=1S/C7H7F2NO/c8-5-2-1-4(3-10)6(9)7(5)11/h1-2,11H,3,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Competitive inhibition of GABA aminotransferase (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
4-aminobutyrate aminotransferase, mitochondrial
(Homo sapiens (Human)) | BDBM50073151
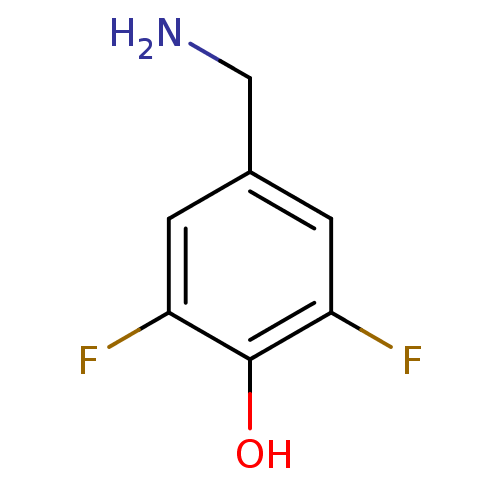
(4-(ammoniomethyl)-2,6-difluorobenzenolate | 4-Amin...)Show InChI InChI=1S/C7H7F2NO/c8-5-1-4(3-10)2-6(9)7(5)11/h1-2,11H,3,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Competitive inhibition of GABA aminotransferase (unknown origin) |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50340481
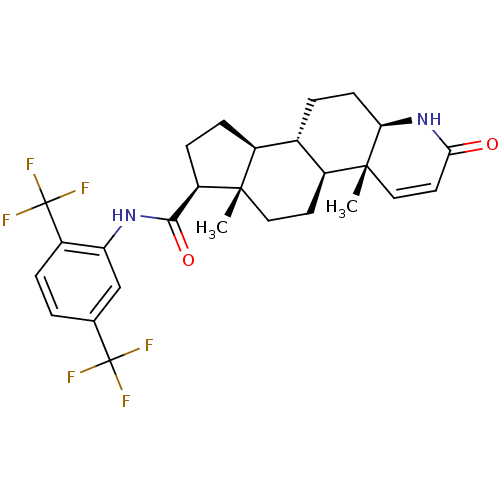
(Avodart | CHEMBL1200969 | DUTASTERIDE | GG-745 | G...)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@H]4NC(=O)C=C[C@]34C)[C@@H]1CC[C@@H]2C(=O)Nc1cc(ccc1C(F)(F)F)C(F)(F)F |r,c:12| Show InChI InChI=1S/C27H30F6N2O2/c1-24-11-9-17-15(4-8-21-25(17,2)12-10-22(36)35-21)16(24)6-7-19(24)23(37)34-20-13-14(26(28,29)30)3-5-18(20)27(31,32)33/h3,5,10,12-13,15-17,19,21H,4,6-9,11H2,1-2H3,(H,34,37)(H,35,36)/t15-,16-,17-,19+,21+,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human type 2 5alpha reductase |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50060440
(3-[3-(4-Benzyl-piperazin-1-yl)-propyl]-5-[1,2,4]tr...)Show SMILES C(CN1CCN(Cc2ccccc2)CC1)Cc1c[nH]c2ccc(cc12)-n1cnnc1 Show InChI InChI=1S/C24H28N6/c1-2-5-20(6-3-1)17-29-13-11-28(12-14-29)10-4-7-21-16-25-24-9-8-22(15-23(21)24)30-18-26-27-19-30/h1-3,5-6,8-9,15-16,18-19,25H,4,7,10-14,17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human 5HT1D expressed in CHO cells by [35S]-GTPgammaS binding assay |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data