

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Bos taurus) | BDBM50059852 ((S)-2-((S)-2-((S)-2-((R)-2-amino-3-mercaptopropyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Cold Spring Harbor Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for concentration that caused 50% inhibition of bovine Farnesyltransferase | Bioorg Med Chem Lett 9: 847-52 (1999) BindingDB Entry DOI: 10.7270/Q21Z43KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50059852 ((S)-2-((S)-2-((S)-2-((R)-2-amino-3-mercaptopropyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Cold Spring Harbor Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for concentration that caused 50% inhibition of yeast Farnesyltransferase (expressed as a GST fusion protein in E. coli) | Bioorg Med Chem Lett 9: 847-52 (1999) BindingDB Entry DOI: 10.7270/Q21Z43KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50076045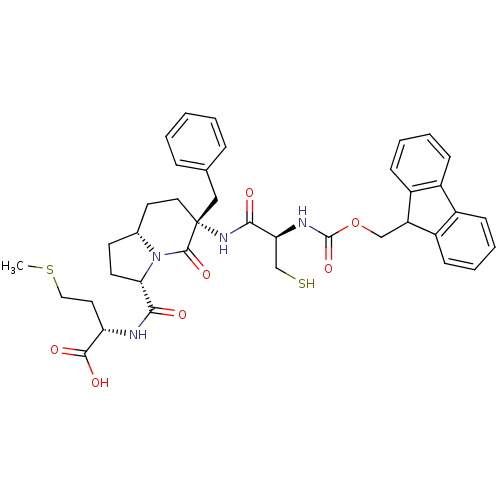 ((S)-2-({(3S,6R,8aR)-6-Benzyl-6-[(R)-2-(9H-fluoren-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cold Spring Harbor Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for concentration that caused 50% inhibition of yeast Farnesyltransferase (expressed as a GST fusion protein in E. coli) | Bioorg Med Chem Lett 9: 847-52 (1999) BindingDB Entry DOI: 10.7270/Q21Z43KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50076042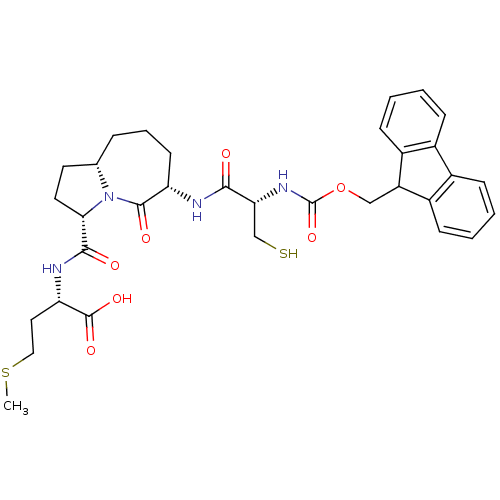 ((S)-2-({(3S,6S,9aS)-6-[(S)-2-(9H-Fluoren-9-ylmetho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cold Spring Harbor Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for concentration that caused 50% inhibition of yeast Farnesyltransferase (expressed as a GST fusion protein in E. coli) | Bioorg Med Chem Lett 9: 847-52 (1999) BindingDB Entry DOI: 10.7270/Q21Z43KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Bos taurus) | BDBM50076043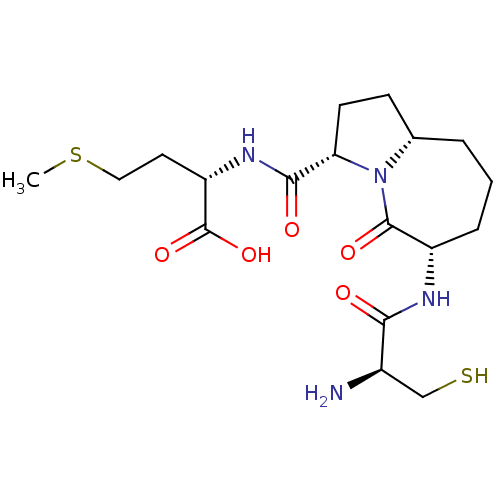 ((S)-2-{[(3S,6S,9aS)-6-((S)-2-Amino-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cold Spring Harbor Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for concentration that caused 50% inhibition of bovine Farnesyltransferase | Bioorg Med Chem Lett 9: 847-52 (1999) BindingDB Entry DOI: 10.7270/Q21Z43KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Bos taurus) | BDBM50076044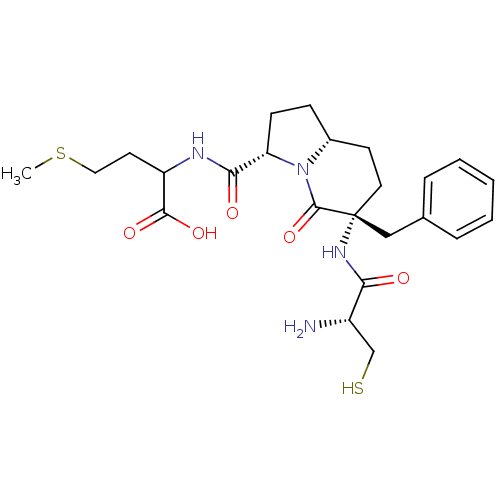 ((S)-2-{[(3S,6R,8aR)-6-((R)-2-Amino-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Cold Spring Harbor Laboratory Curated by ChEMBL | Assay Description Compound was evaluated for concentration that caused 50% inhibition of bovine Farnesyltransferase | Bioorg Med Chem Lett 9: 847-52 (1999) BindingDB Entry DOI: 10.7270/Q21Z43KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||