

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523376 (CHEMBL4589980) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028685 (CHEMBL3356536) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523394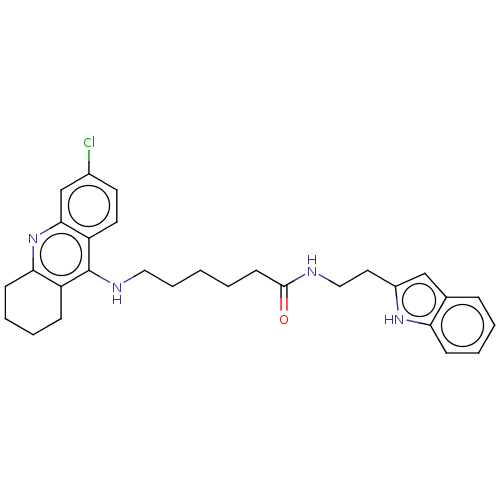 (CHEMBL4448188) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099602 (CHEMBL3343931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523387 (CHEMBL4556281) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523391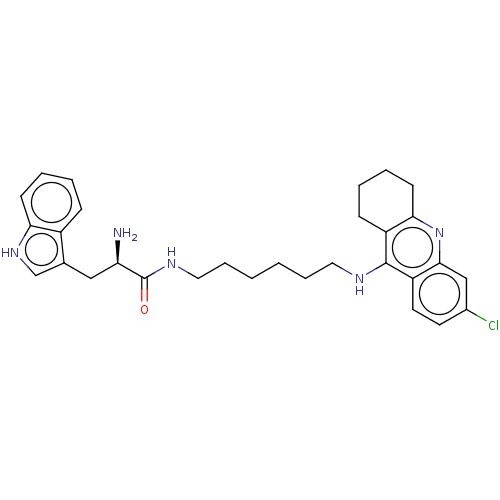 (CHEMBL4449083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523377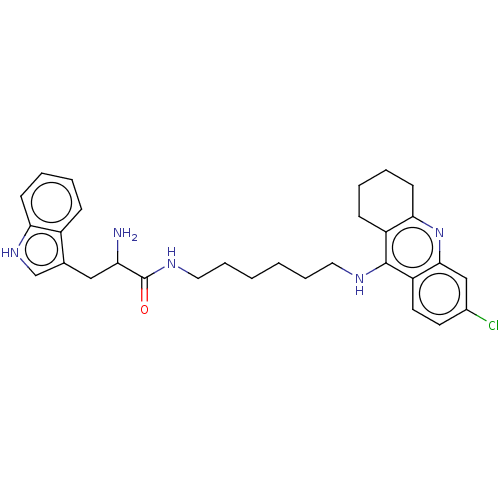 (CHEMBL4559593) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523376 (CHEMBL4589980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523387 (CHEMBL4556281) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523377 (CHEMBL4559593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523375 (CHEMBL4555818) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523388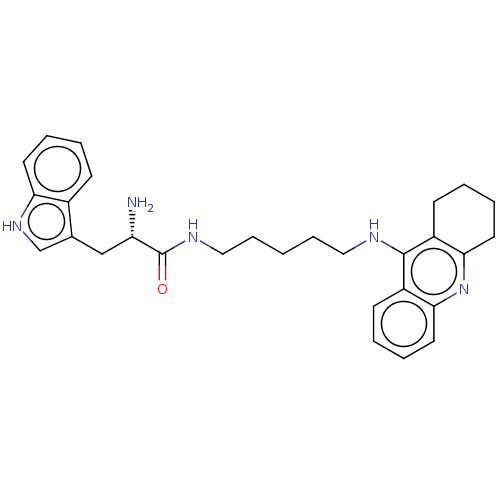 (CHEMBL4556575) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523389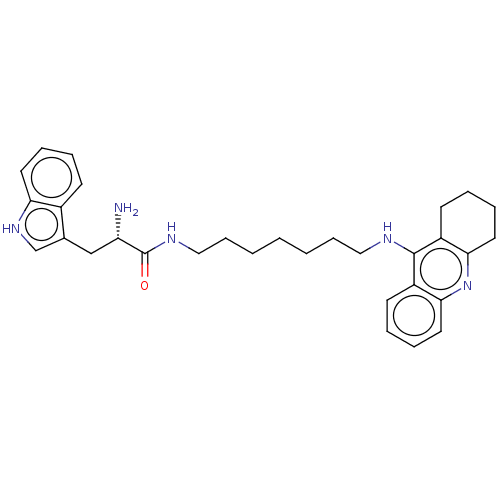 (CHEMBL4559477) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523383 (CHEMBL4438120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523393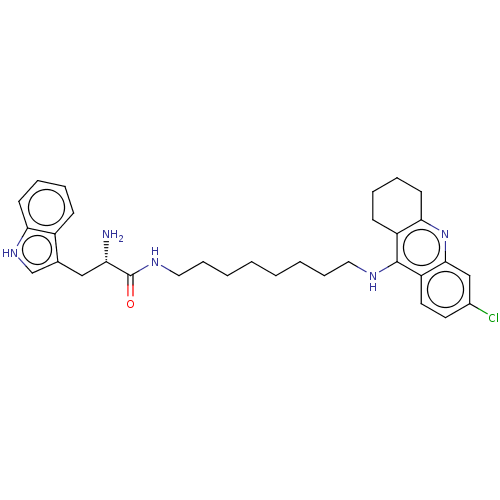 (CHEMBL4466307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523375 (CHEMBL4555818) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523379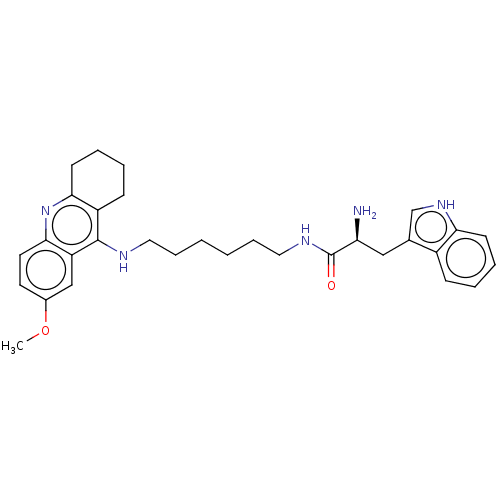 (CHEMBL4467355) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50265253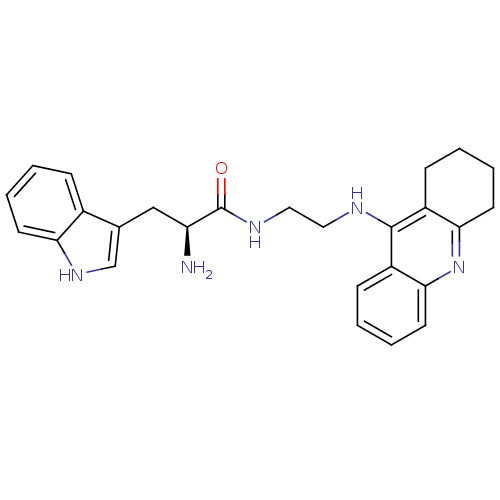 ((S)-2-amino-3-(1H-indol-3-yl)-N-(2-(1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523373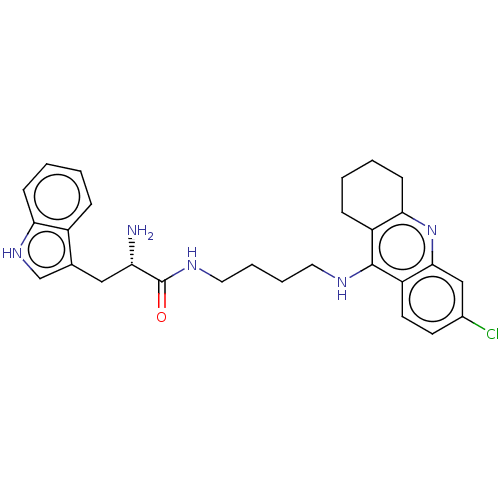 (CHEMBL4552440) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523371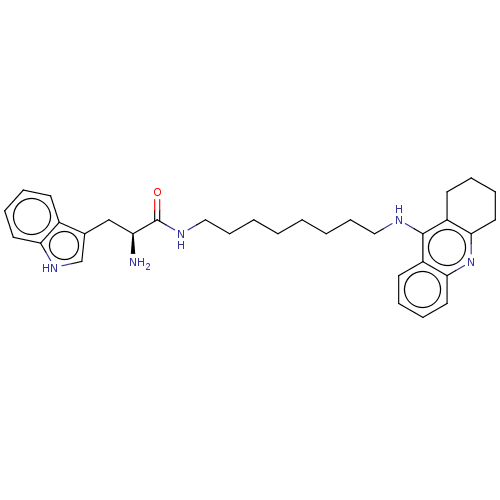 (CHEMBL4456435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523386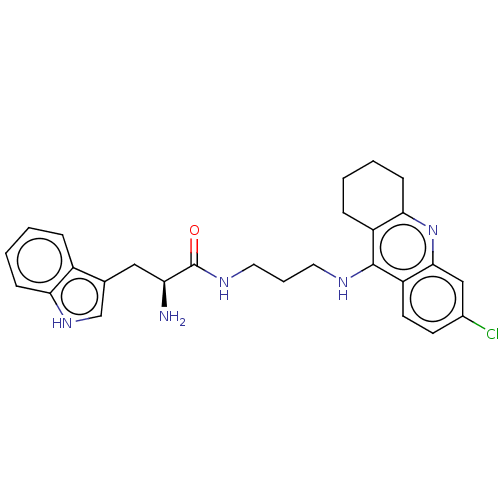 (CHEMBL4541558) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523382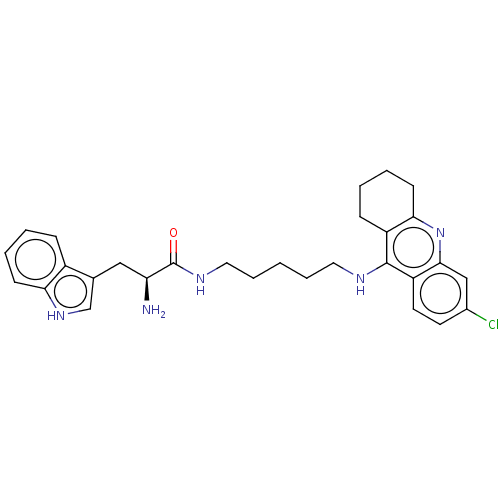 (CHEMBL4563209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523371 (CHEMBL4456435) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523382 (CHEMBL4563209) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523380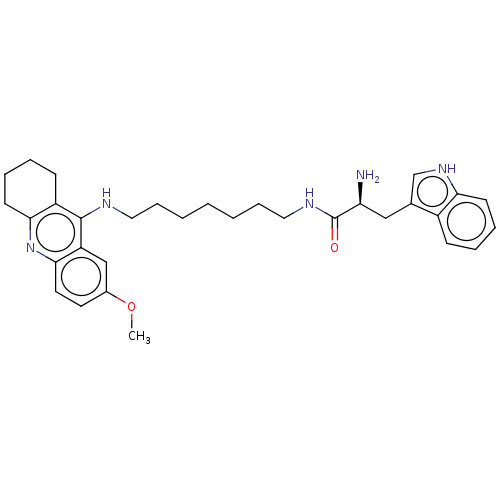 (CHEMBL4586157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523389 (CHEMBL4559477) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523373 (CHEMBL4552440) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50099602 (CHEMBL3343931) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523384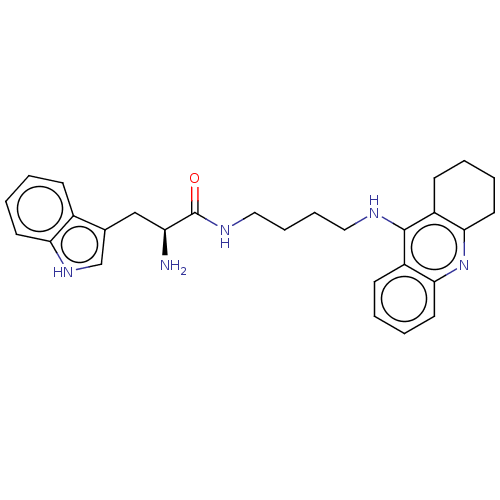 (CHEMBL4459416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523392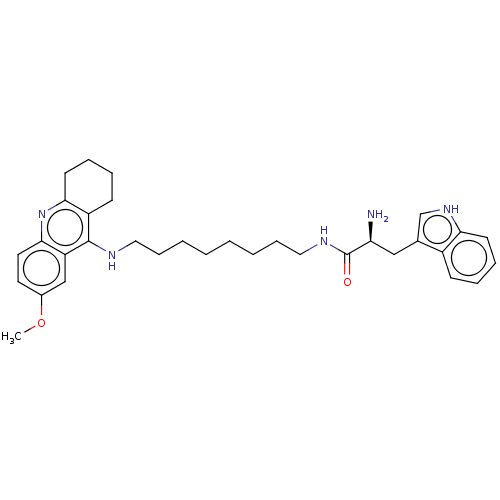 (CHEMBL4582203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523391 (CHEMBL4449083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523393 (CHEMBL4466307) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523386 (CHEMBL4541558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523381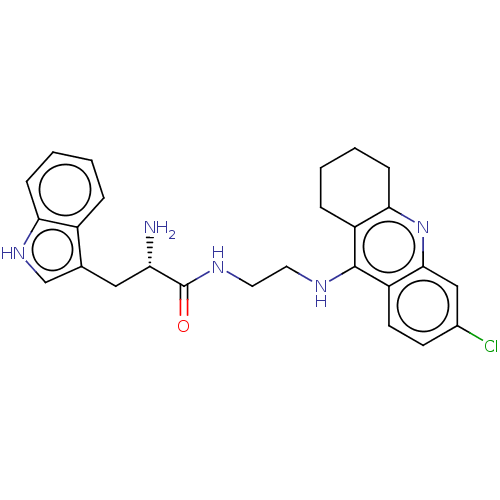 (CHEMBL4444822) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523394 (CHEMBL4448188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523374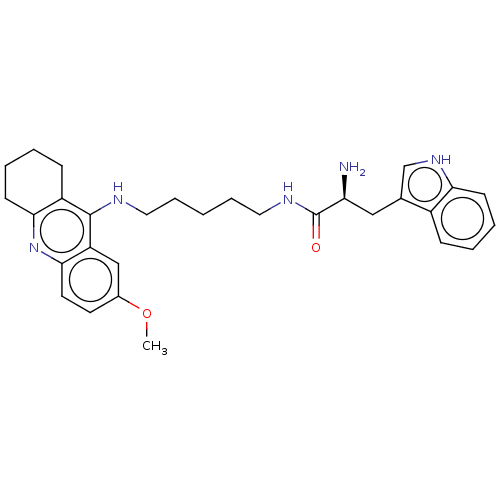 (CHEMBL4472570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523388 (CHEMBL4556575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523381 (CHEMBL4444822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523372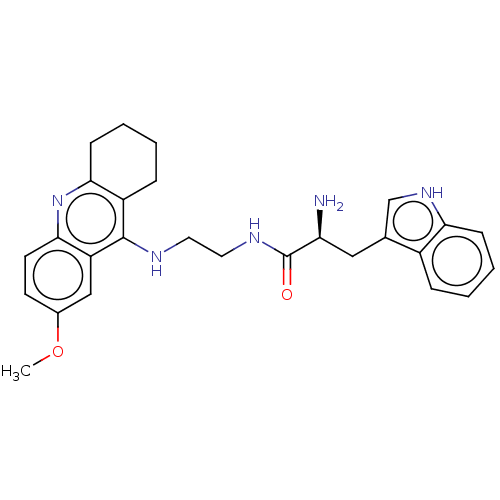 (CHEMBL4538398) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523385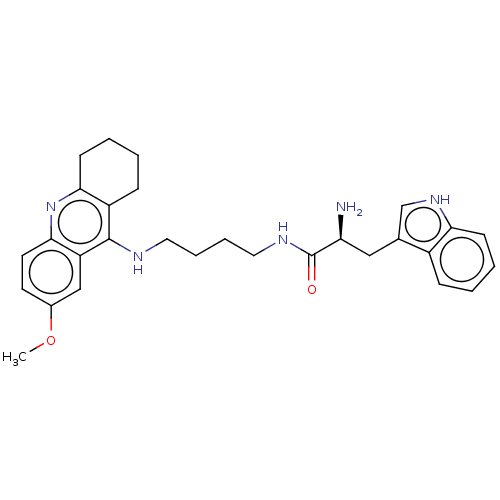 (CHEMBL4475391) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50028685 (CHEMBL3356536) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523383 (CHEMBL4438120) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523374 (CHEMBL4472570) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Rattus norvegicus) | BDBM50209245 (CHEMBL247378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of nNOS in Wistar rat cortical homogenates incubated for 30 mins | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50265253 ((S)-2-amino-3-(1H-indol-3-yl)-N-(2-(1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523379 (CHEMBL4467355) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523380 (CHEMBL4586157) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 85 total ) | Next | Last >> |