

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substra... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524422 (CHEMBL4557635) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substra... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524423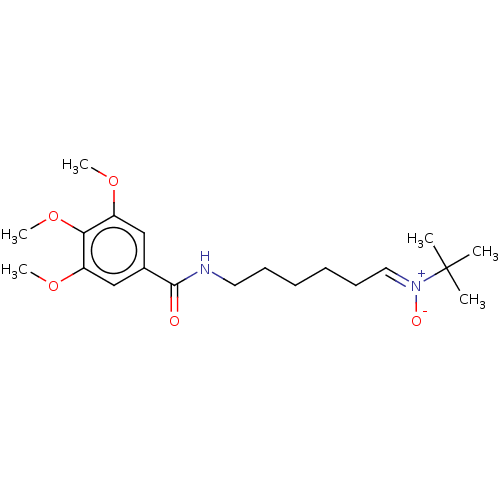 (CHEMBL4456422) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE assessed as inhibition constant using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substra... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured every minute... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured for 5 mins ... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524422 (CHEMBL4557635) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured every minute... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524424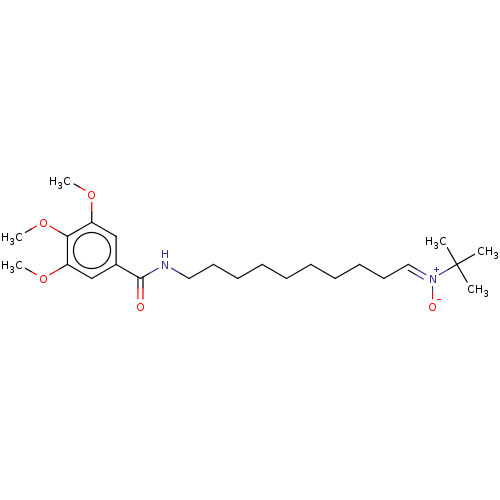 (CHEMBL4452769) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured every minute... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524427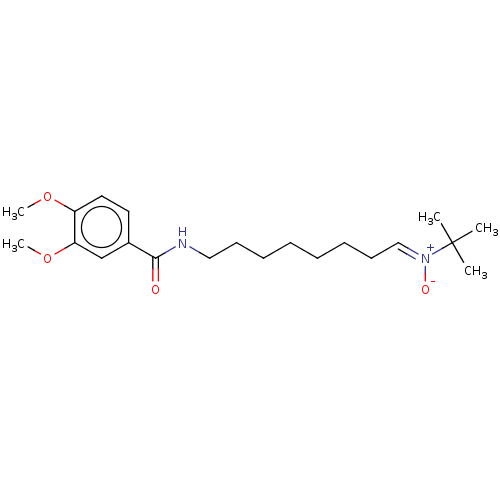 (CHEMBL4555086) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured every minute... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524421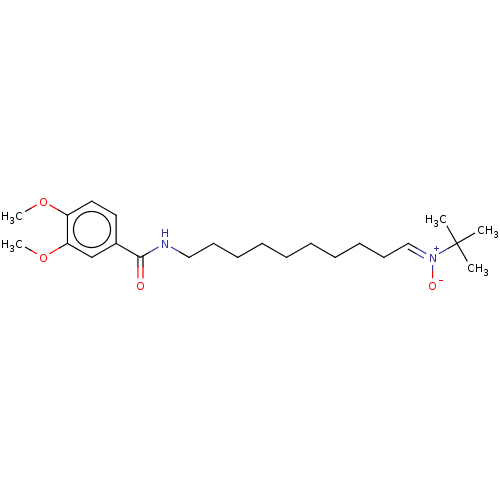 (CHEMBL4575512) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured every minute... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524420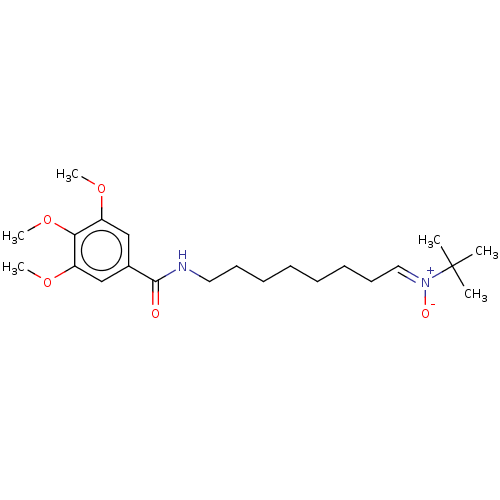 (CHEMBL4460000) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured every minute... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524423 (CHEMBL4456422) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured every minute... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524425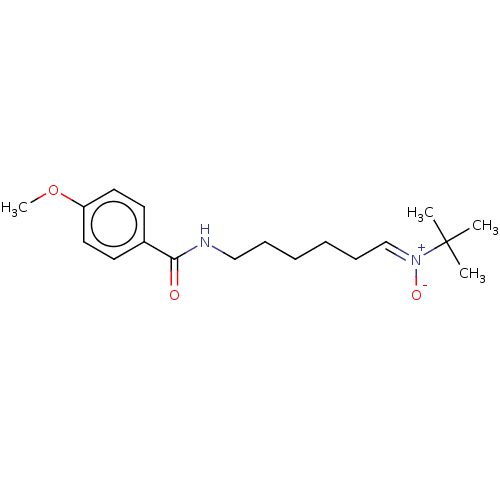 (CHEMBL4564925) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured every minute... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50524426 (CHEMBL4544304) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Porto Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition measured every minute... | Eur J Med Chem 174: 116-129 (2019) Article DOI: 10.1016/j.ejmech.2019.04.026 BindingDB Entry DOI: 10.7270/Q2KW5KFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||