 Found 14 hits Enz. Inhib. hit(s) with all data for entry = 50009047
Found 14 hits Enz. Inhib. hit(s) with all data for entry = 50009047 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50524833

(CHEMBL283631 | NSC-628679)Show SMILES [H][C@]12COC(=O)[C@]1([H])[C@H](c1cc(OC)c(O)c(OC)c1)c1cc3OCOc3cc1[C@H]2Nc1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C27H24N2O9/c1-34-21-7-13(8-22(35-2)26(21)30)23-16-9-19-20(38-12-37-19)10-17(16)25(18-11-36-27(31)24(18)23)28-14-3-5-15(6-4-14)29(32)33/h3-10,18,23-25,28,30H,11-12H2,1-2H3/t18-,23+,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1alpha in human U373 cells |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
Egl nine homolog 1
(Homo sapiens (Human)) | BDBM50510124

(CHEMBL3186774)Show SMILES OC(=O)CNC(=O)c1c(O)c2ccccc2n(Cc2ccccc2)c1=O Show InChI InChI=1S/C19H16N2O5/c22-15(23)10-20-18(25)16-17(24)13-8-4-5-9-14(13)21(19(16)26)11-12-6-2-1-3-7-12/h1-9,24H,10-11H2,(H,20,25)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human PHD2 |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50524837
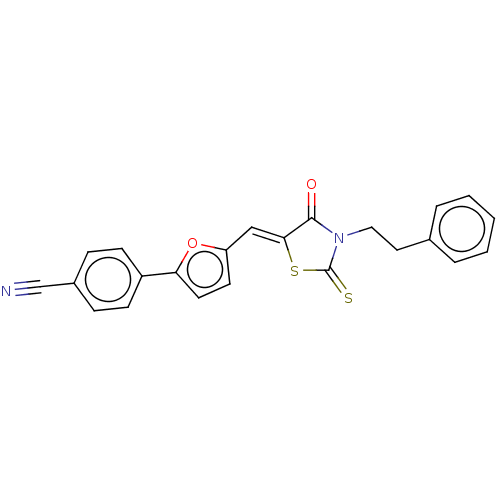
(CHEMBL4441544)Show SMILES O=C1N(CCc2ccccc2)C(=S)S\C1=C/c1ccc(o1)-c1ccc(cc1)C#N Show InChI InChI=1S/C23H16N2O2S2/c24-15-17-6-8-18(9-7-17)20-11-10-19(27-20)14-21-22(26)25(23(28)29-21)13-12-16-4-2-1-3-5-16/h1-11,14H,12-13H2/b21-14- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p300 (unknown origin)/CBP (unknown origin) protein-protein interaction |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50524835
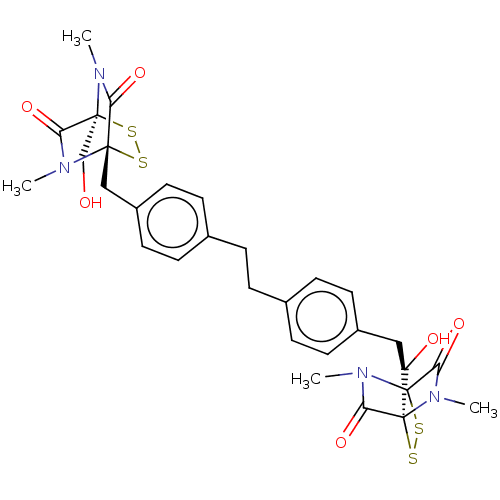
(CHEMBL4468788)Show SMILES CN1C(=O)[C@]2(Cc3ccc(CCc4ccc(C[C@]56SS[C@](CO)(N(C)C5=O)C(=O)N6C)cc4)cc3)SS[C@@]1(CO)C(=O)N2C |r,TLB:41:40:1.2:36.35,26:25:29.27:19.18,3:2:42.40:36.35,30:29:23.25:19.18,THB:28:27:23.25:19.18,24:23:29.27:19.18,0:1:42.40:36.35,43:42:1.2:36.35| Show InChI InChI=1S/C30H34N4O6S4/c1-31-25(39)29(17-35)33(3)23(37)27(31,41-43-29)15-21-11-7-19(8-12-21)5-6-20-9-13-22(14-10-20)16-28-24(38)34(4)30(18-36,44-42-28)26(40)32(28)2/h7-14,35-36H,5-6,15-18H2,1-4H3/t27-,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p300 (unknown origin)/CBP (unknown origin) protein-protein interaction by fluorescence polarization assay |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
von Hippel-Lindau disease tumor suppressor
(Homo sapiens (Human)) | BDBM50014097
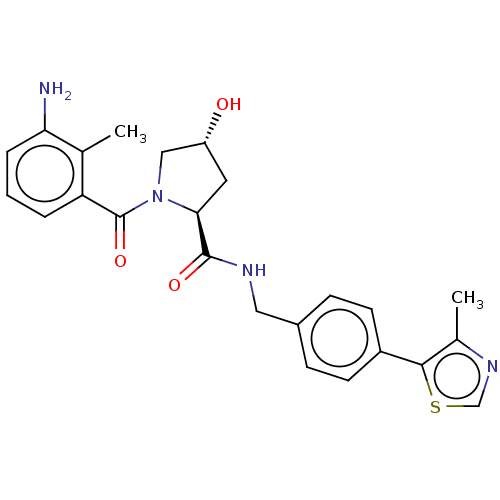
(CHEMBL3260851)Show SMILES Cc1ncsc1-c1ccc(CNC(=O)[C@@H]2C[C@@H](O)CN2C(=O)c2cccc(N)c2C)cc1 |r| Show InChI InChI=1S/C24H26N4O3S/c1-14-19(4-3-5-20(14)25)24(31)28-12-18(29)10-21(28)23(30)26-11-16-6-8-17(9-7-16)22-15(2)27-13-32-22/h3-9,13,18,21,29H,10-12,25H2,1-2H3,(H,26,30)/t18-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of VHL (unknown origin) by fluorescence polarization assay |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50524836

(CHEMBL4555267)Show SMILES CN1C(=O)[C@]2(Cc3ccc(C[C@]45SS[C@](CO)(N(C)C4=O)C(=O)N5C)cc3)SS[C@@]1(CO)C(=O)N2C |r,TLB:20:19:13.12:21.23,33:32:27.28:1.2,24:23:13.12:17.19,3:2:27.28:34.32,THB:0:1:27.28:34.32,35:34:27.28:1.2,18:17:13.12:21.23,22:21:13.12:17.19| Show InChI InChI=1S/C22H26N4O6S4/c1-23-17(31)21(11-27)25(3)15(29)19(23,33-35-21)9-13-5-7-14(8-6-13)10-20-16(30)26(4)22(12-28,36-34-20)18(32)24(20)2/h5-8,27-28H,9-12H2,1-4H3/t19-,20-,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p300 (unknown origin)/CBP (unknown origin) protein-protein interaction by fluorescence polarization assay |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM76423

(2-(4-methylphenyl)-2-oxoethyl 3-({2,4-dinitrobenzo...)Show SMILES Cc1ccc(cc1)C(=O)COC(=O)c1cccc(NC(=O)c2ccc(cc2[N+]([O-])=O)[N+]([O-])=O)c1 Show InChI InChI=1S/C23H17N3O8/c1-14-5-7-15(8-6-14)21(27)13-34-23(29)16-3-2-4-17(11-16)24-22(28)19-10-9-18(25(30)31)12-20(19)26(32)33/h2-12H,13H2,1H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1alpha in human HeLa cells after 6 hrs by luciferase reporter gene assay |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50315537
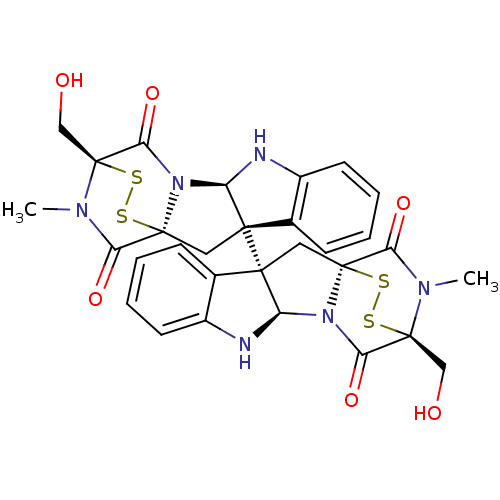
(CHEMBL1089316 | chaetocin)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(CO)SS3)[C@]12C[C@]34SS[C@](CO)(N(C)C3=O)C(=O)N4[C@H]1Nc1ccccc21 |r,THB:36:35:31.33:26.27,7:15:1.2:22.21,38:37:31.33:26.27,17:16:1.2:22.21| Show InChI InChI=1S/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40)34(2)30(14-38,46-44-28)24(42)36(28)20(26)32-18-10-6-4-8-16(18)26/h3-10,19-20,31-32,37-38H,11-14H2,1-2H3/t19-,20-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal biotinylated HIF-1alpha (786 to 826 residues) (unknown origin)/GST-tagged p300-CH1 domain (unknown origin) protein-protein i... |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300/Hypoxia-inducible factor 1-alpha
(Homo sapiens (Human)) | BDBM50524838
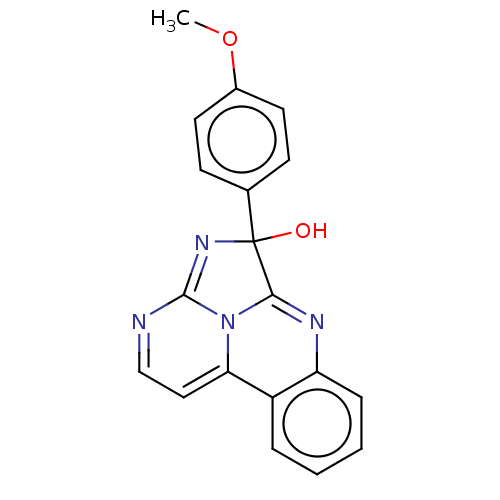
(CHEMBL4458726)Show SMILES COc1ccc(cc1)C1(O)N=C2N=CC=C3N2C1=Nc1ccccc31 |c:13,15,t:11,20| Show InChI InChI=1S/C19H14N4O2/c1-25-13-8-6-12(7-9-13)19(24)17-21-15-5-3-2-4-14(15)16-10-11-20-18(22-19)23(16)17/h2-11,24H,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIF-1alpha- CTAD domain (unknown origin)/p300-CH1 domain (unknown origin) protein-protein interaction |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
CREB-binding protein/Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50485362
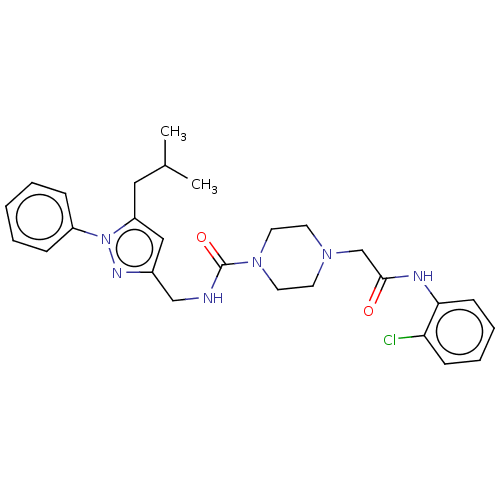
(CHEMBL2057226)Show SMILES Cl.CC(C)Cc1cc(CNC(=O)N2CCN(CC(=O)Nc3ccccc3Cl)CC2)nn1-c1ccccc1 Show InChI InChI=1S/C27H33ClN6O2/c1-20(2)16-23-17-21(31-34(23)22-8-4-3-5-9-22)18-29-27(36)33-14-12-32(13-15-33)19-26(35)30-25-11-7-6-10-24(25)28/h3-11,17,20H,12-16,18-19H2,1-2H3,(H,29,36)(H,30,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged p300 (unknown origin)/CBP (unknown origin) protein-protein interaction |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50524834
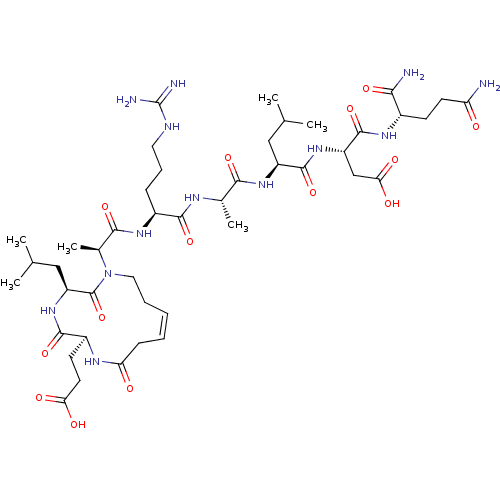
(CHEMBL4435255)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)N1CC\C=C/CC(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O |r,c:29| Show InChI InChI=1S/C44H73N13O14/c1-22(2)19-29(41(69)55-30(21-35(62)63)42(70)52-26(36(46)64)13-15-32(45)58)54-37(65)24(5)50-39(67)27(11-10-17-49-44(47)48)53-38(66)25(6)57-18-9-7-8-12-33(59)51-28(14-16-34(60)61)40(68)56-31(43(57)71)20-23(3)4/h7-8,22-31H,9-21H2,1-6H3,(H2,45,58)(H2,46,64)(H,50,67)(H,51,59)(H,52,70)(H,53,66)(H,54,65)(H,55,69)(H,56,68)(H,60,61)(H,62,63)(H4,47,48,49)/b8-7-/t24-,25-,26-,27-,28-,29-,30-,31-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to p300 (unknown origin) |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |
Hypoxia-inducible factor 1-alpha inhibitor
(Homo sapiens (Human)) | BDBM26107
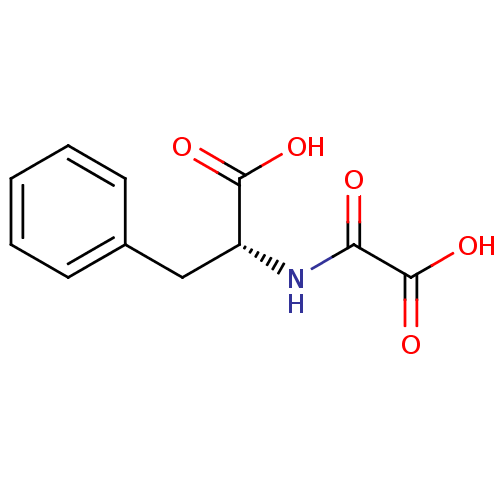
((2R)-2-(formamidoformic acid)-3-phenylpropanoic ac...)Show InChI InChI=1S/C11H11NO5/c13-9(11(16)17)12-8(10(14)15)6-7-4-2-1-3-5-7/h1-5,8H,6H2,(H,12,13)(H,14,15)(H,16,17)/t8-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 8.30E+4 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of FIH (unknown origin) |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
von Hippel-Lindau disease tumor suppressor
(Homo sapiens (Human)) | BDBM50459944
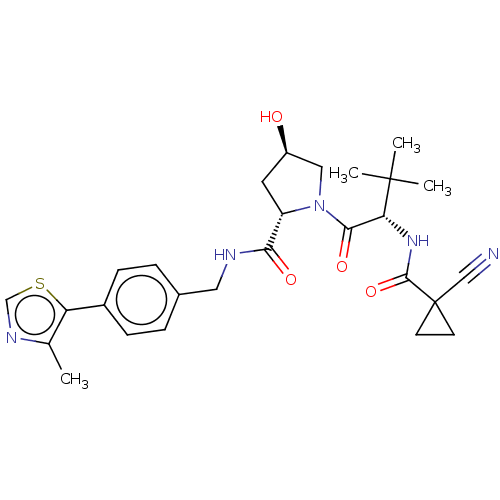
(CHEMBL4225537)Show SMILES Cc1ncsc1-c1ccc(CNC(=O)[C@@H]2C[C@@H](O)CN2C(=O)[C@@H](NC(=O)C2(CC2)C#N)C(C)(C)C)cc1 |r| Show InChI InChI=1S/C27H33N5O4S/c1-16-21(37-15-30-16)18-7-5-17(6-8-18)12-29-23(34)20-11-19(33)13-32(20)24(35)22(26(2,3)4)31-25(36)27(14-28)9-10-27/h5-8,15,19-20,22,33H,9-13H2,1-4H3,(H,29,34)(H,31,36)/t19-,20+,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to VHL (unknown origin) |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50524839

(CHEMBL4466328)Show SMILES CC(C)C[C@H](N1CCN[C@@H](CC(C)C)C1=O)C(=O)N1CCN([C@@H](CCC(N)=O)C(N)=O)C(=O)[C@@H]1C |r| Show InChI InChI=1S/C24H42N6O5/c1-14(2)12-17-23(34)29(9-8-27-17)19(13-15(3)4)24(35)28-10-11-30(22(33)16(28)5)18(21(26)32)6-7-20(25)31/h14-19,27H,6-13H2,1-5H3,(H2,25,31)(H2,26,32)/t16-,17-,18-,19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University
Curated by ChEMBL
| Assay Description
Binding affinity to p300-CH1 domain (unknown origin) |
J Med Chem 62: 5725-5749 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01596
BindingDB Entry DOI: 10.7270/Q2QF8X9V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data