

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50528693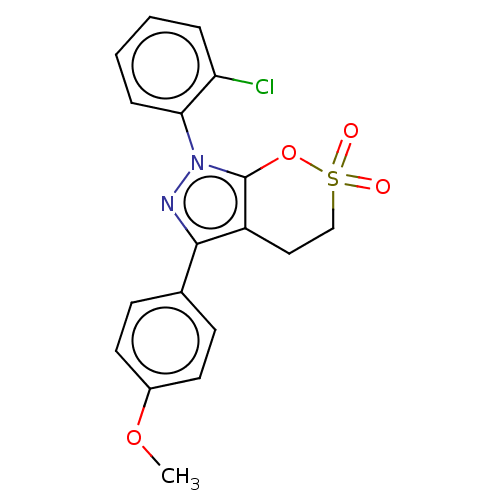 (CHEMBL4513389) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using varying levels of butyrylthiocholine iodide as substrate by Line weaver Burk reciprocal plot analysis | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528690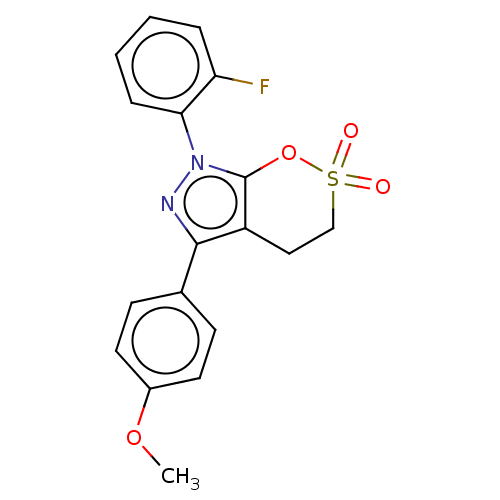 (CHEMBL4545271) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BChE using varying levels of butyrylthiocholine iodide as substrate by Line weaver Burk reciprocal plot analysis | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by El... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528685 (CHEMBL4457213) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528693 (CHEMBL4513389) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528690 (CHEMBL4545271) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528694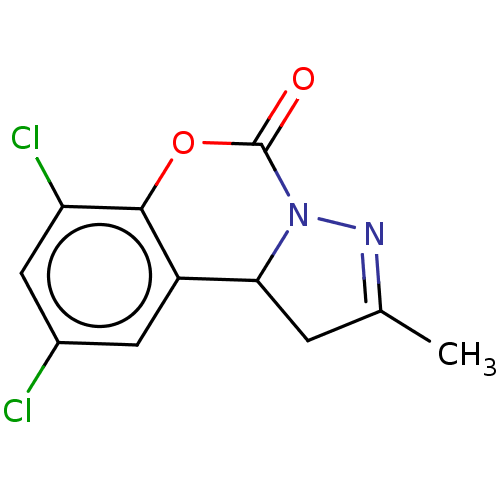 (CHEMBL4440216) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528684 (CHEMBL4560302) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528682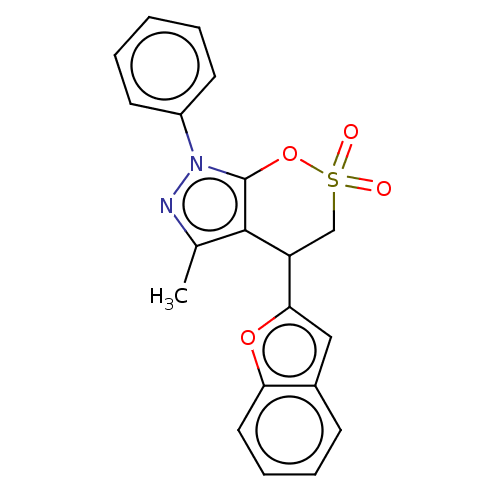 (CHEMBL4461045) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528699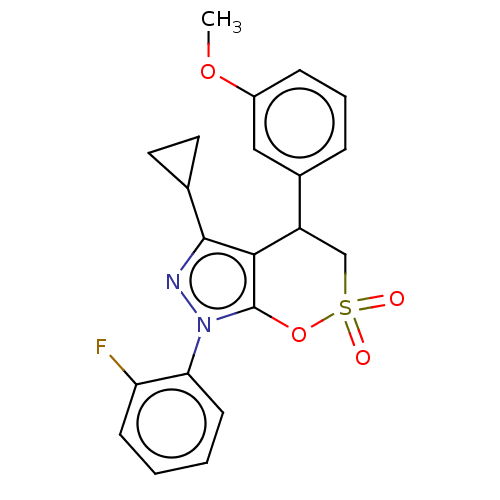 (CHEMBL4549779) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528678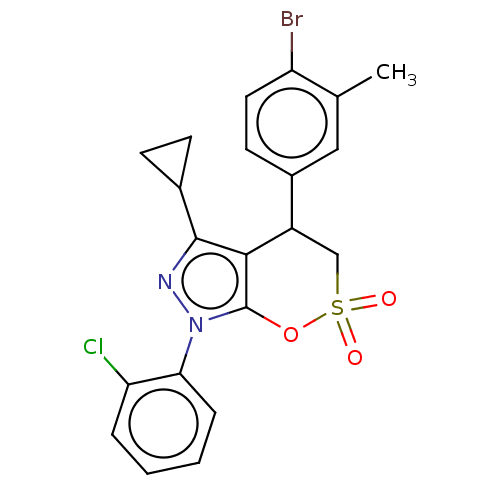 (CHEMBL4539779) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528701 (CHEMBL4464316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528687 (CHEMBL4587583) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528691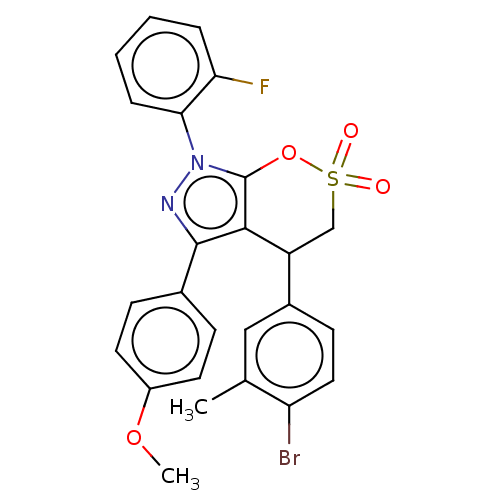 (CHEMBL4556638) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528689 (CHEMBL4443049) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528702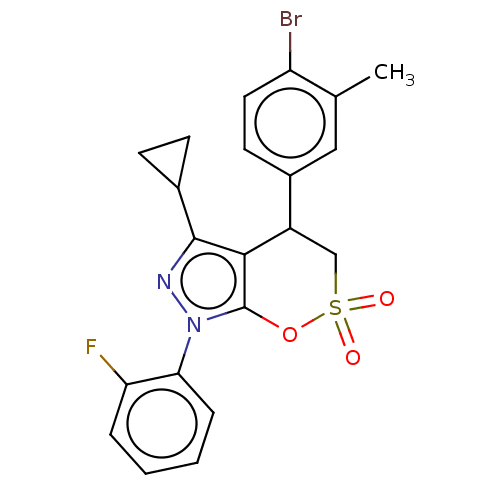 (CHEMBL4470686) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528696 (CHEMBL4451107) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528695 (CHEMBL4522656) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528686 (CHEMBL4452132) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528679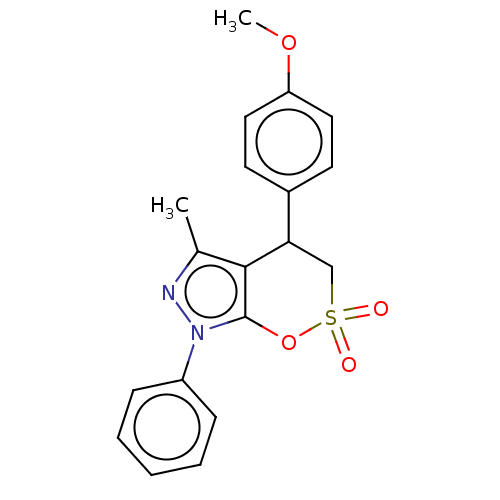 (CHEMBL4463401) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528680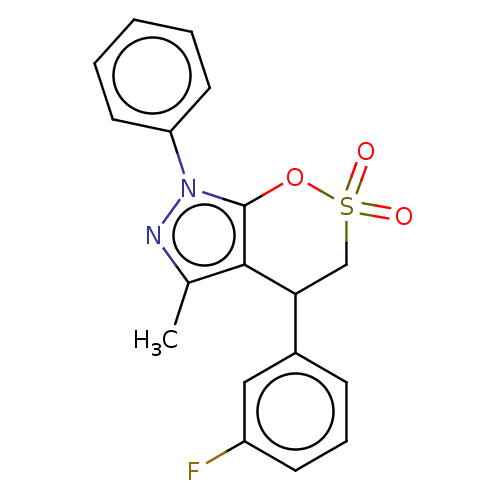 (CHEMBL4536663) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528683 (CHEMBL4448386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528698 (CHEMBL4565000) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528697 (CHEMBL4463274) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528700 (CHEMBL4466602) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528688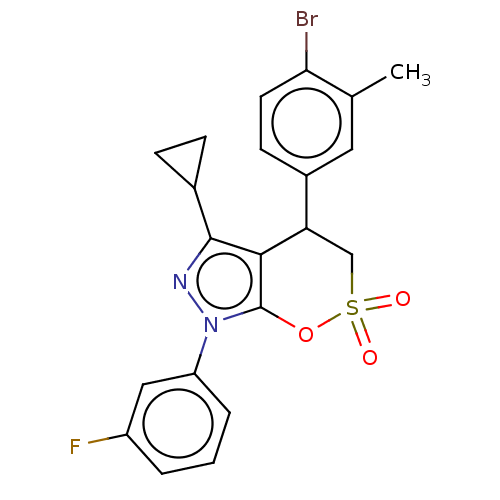 (CHEMBL4547095) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528692 (CHEMBL4565603) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528703 (CHEMBL4574172) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50528697 (CHEMBL4463274) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by El... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528705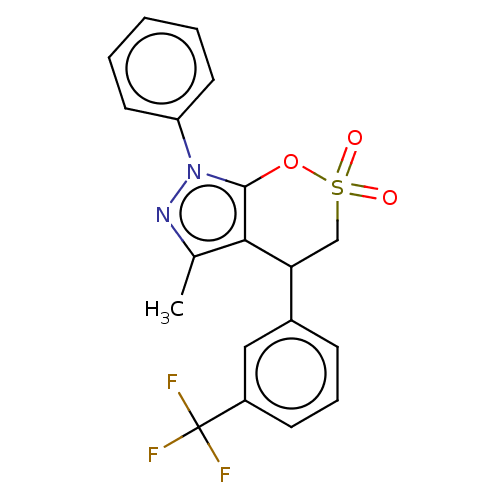 (CHEMBL4445890) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528681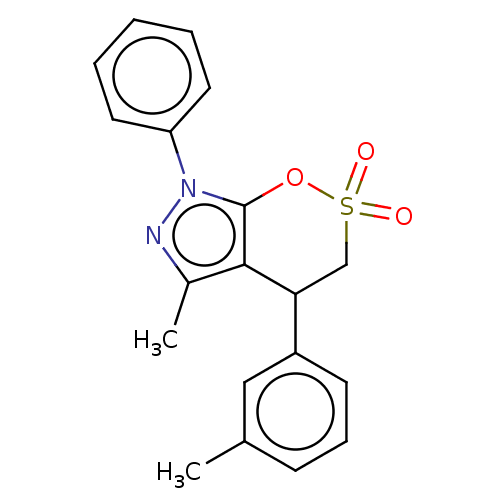 (CHEMBL4470984) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528677 (CHEMBL4449553) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50528694 (CHEMBL4440216) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by El... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50528704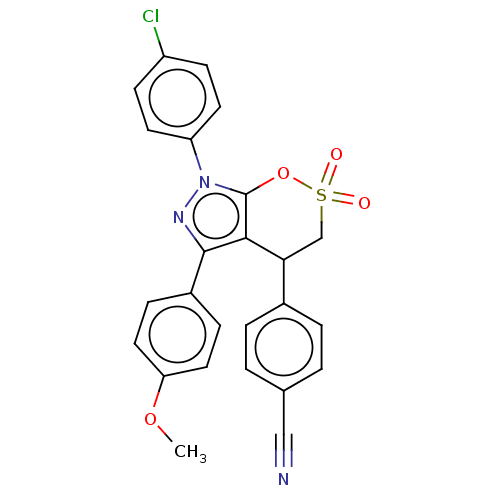 (CHEMBL4468896) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by ... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins followed by substrate addition by El... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111598 BindingDB Entry DOI: 10.7270/Q24F1V59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||