 Found 56 hits Enz. Inhib. hit(s) with all data for entry = 50009425
Found 56 hits Enz. Inhib. hit(s) with all data for entry = 50009425 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528779
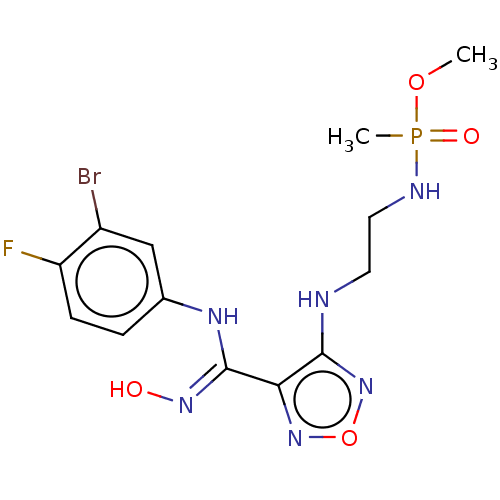
(CHEMBL4514196)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C13H17BrFN6O4P/c1-24-26(2,23)17-6-5-16-12-11(20-25-21-12)13(19-22)18-8-3-4-10(15)9(14)7-8/h3-4,7,22H,5-6H2,1-2H3,(H,16,21)(H,17,23)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528775
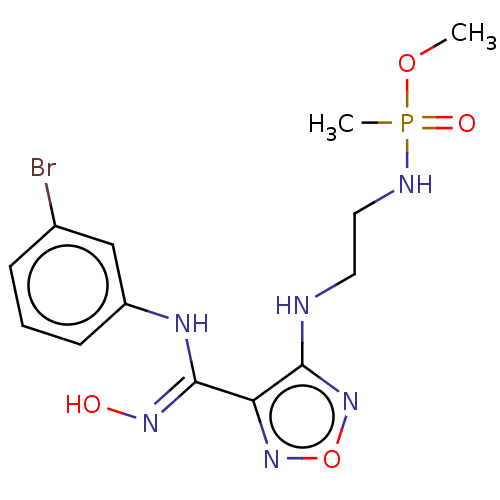
(CHEMBL4552649)Show InChI InChI=1S/C13H18BrN6O4P/c1-23-25(2,22)16-7-6-15-12-11(19-24-20-12)13(18-21)17-10-5-3-4-9(14)8-10/h3-5,8,21H,6-7H2,1-2H3,(H,15,20)(H,16,22)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528774
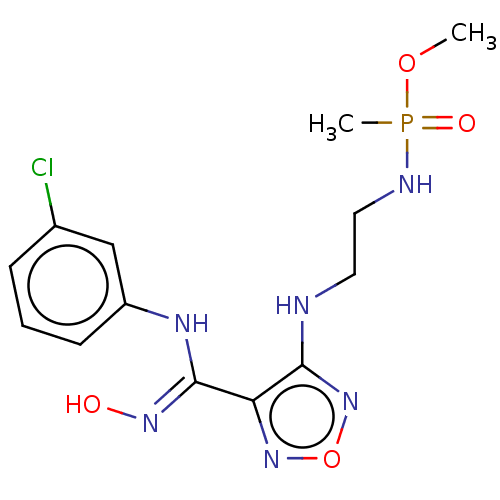
(CHEMBL4457875)Show InChI InChI=1S/C13H18ClN6O4P/c1-23-25(2,22)16-7-6-15-12-11(19-24-20-12)13(18-21)17-10-5-3-4-9(14)8-10/h3-5,8,21H,6-7H2,1-2H3,(H,15,20)(H,16,22)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528762
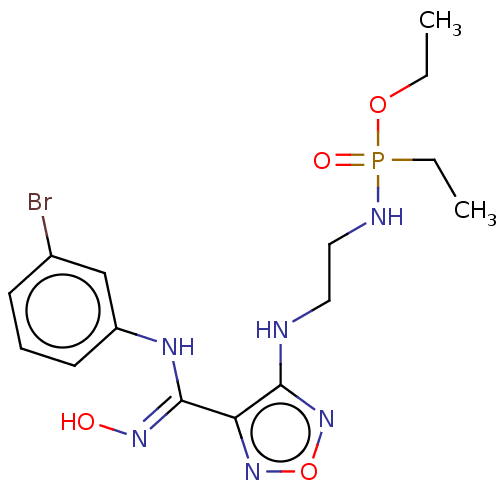
(CHEMBL4581189)Show SMILES CCOP(=O)(CC)NCCNc1nonc1\C(Nc1cccc(Br)c1)=N\O Show InChI InChI=1S/C15H22BrN6O4P/c1-3-25-27(24,4-2)18-9-8-17-14-13(21-26-22-14)15(20-23)19-12-7-5-6-11(16)10-12/h5-7,10,23H,3-4,8-9H2,1-2H3,(H,17,22)(H,18,24)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528761
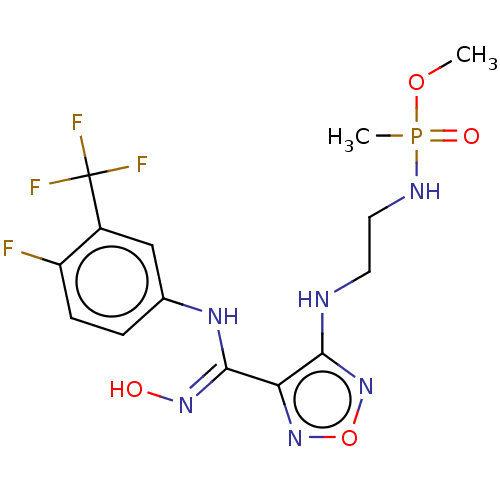
(CHEMBL4552082)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(c1)C(F)(F)F)=N\O Show InChI InChI=1S/C14H17F4N6O4P/c1-27-29(2,26)20-6-5-19-12-11(23-28-24-12)13(22-25)21-8-3-4-10(15)9(7-8)14(16,17)18/h3-4,7,25H,5-6H2,1-2H3,(H,19,24)(H,20,26)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528773
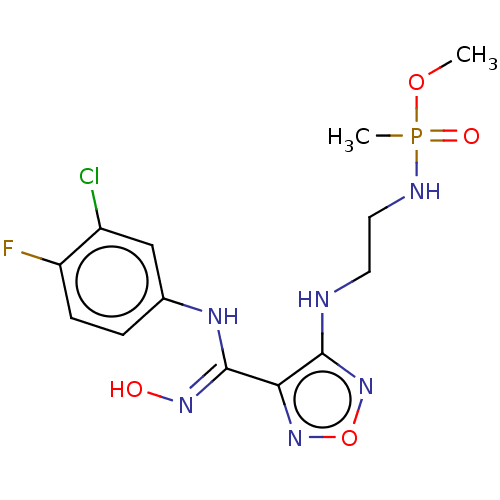
(CHEMBL4447280)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Cl)c1)=N\O Show InChI InChI=1S/C13H17ClFN6O4P/c1-24-26(2,23)17-6-5-16-12-11(20-25-21-12)13(19-22)18-8-3-4-10(15)9(14)7-8/h3-4,7,22H,5-6H2,1-2H3,(H,16,21)(H,17,23)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528780

(CHEMBL4436582)Show SMILES COP(C)(=O)NCCCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C15H21BrFN6O4P/c1-26-28(2,25)19-8-4-3-7-18-14-13(22-27-23-14)15(21-24)20-10-5-6-12(17)11(16)9-10/h5-6,9,24H,3-4,7-8H2,1-2H3,(H,18,23)(H,19,25)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528760
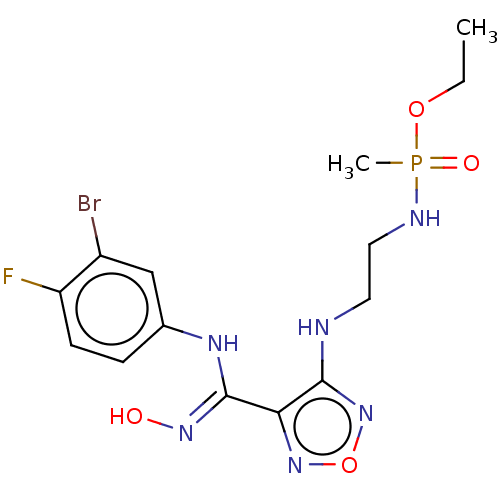
(CHEMBL4444787)Show SMILES CCOP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C14H19BrFN6O4P/c1-3-25-27(2,24)18-7-6-17-13-12(21-26-22-13)14(20-23)19-9-4-5-11(16)10(15)8-9/h4-5,8,23H,3,6-7H2,1-2H3,(H,17,22)(H,18,24)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528763
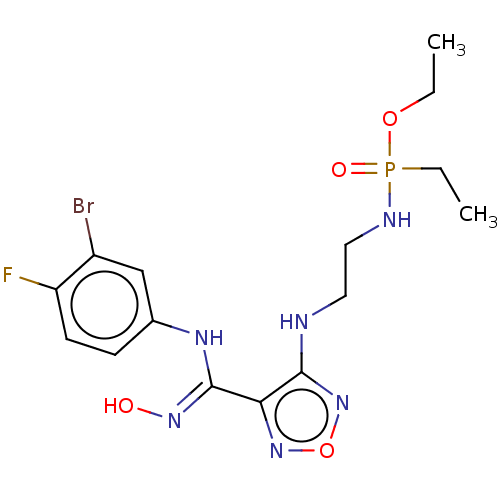
(CHEMBL4472697)Show SMILES CCOP(=O)(CC)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C15H21BrFN6O4P/c1-3-26-28(25,4-2)19-8-7-18-14-13(22-27-23-14)15(21-24)20-10-5-6-12(17)11(16)9-10/h5-6,9,24H,3-4,7-8H2,1-2H3,(H,18,23)(H,19,25)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528783
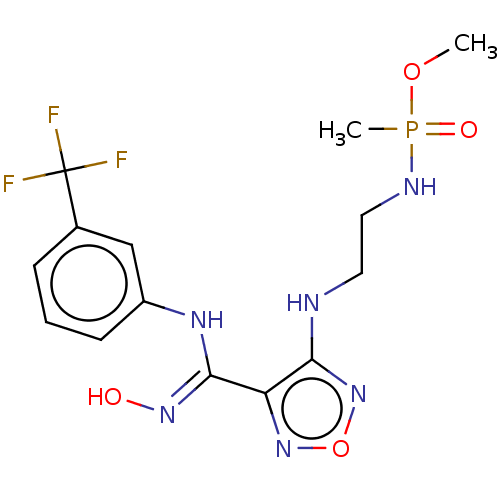
(CHEMBL4455785)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1cccc(c1)C(F)(F)F)=N\O Show InChI InChI=1S/C14H18F3N6O4P/c1-26-28(2,25)19-7-6-18-12-11(22-27-23-12)13(21-24)20-10-5-3-4-9(8-10)14(15,16)17/h3-5,8,24H,6-7H2,1-2H3,(H,18,23)(H,19,25)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528789

(CHEMBL4473190)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(C)c1)=N\O Show InChI InChI=1S/C14H20FN6O4P/c1-9-8-10(4-5-11(9)15)18-14(19-22)12-13(21-25-20-12)16-6-7-17-26(3,23)24-2/h4-5,8,22H,6-7H2,1-3H3,(H,16,21)(H,17,23)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528777
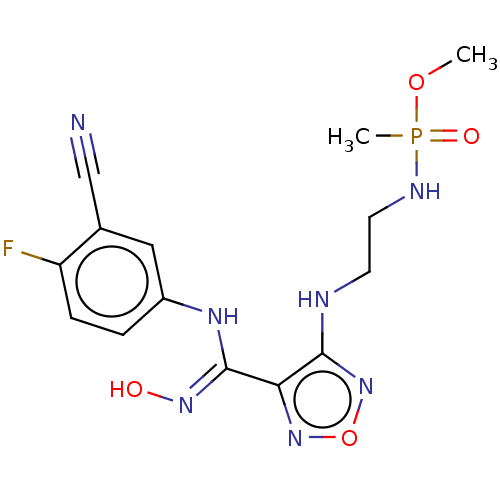
(CHEMBL4455008)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(c1)C#N)=N\O Show InChI InChI=1S/C14H17FN7O4P/c1-25-27(2,24)18-6-5-17-13-12(21-26-22-13)14(20-23)19-10-3-4-11(15)9(7-10)8-16/h3-4,7,23H,5-6H2,1-2H3,(H,17,22)(H,18,24)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528782
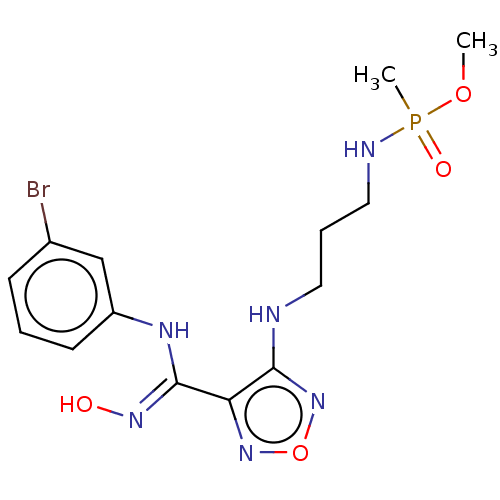
(CHEMBL4454093)Show SMILES COP(C)(=O)NCCCNc1nonc1\C(Nc1cccc(Br)c1)=N\O Show InChI InChI=1S/C14H20BrN6O4P/c1-24-26(2,23)17-8-4-7-16-13-12(20-25-21-13)14(19-22)18-11-6-3-5-10(15)9-11/h3,5-6,9,22H,4,7-8H2,1-2H3,(H,16,21)(H,17,23)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528785
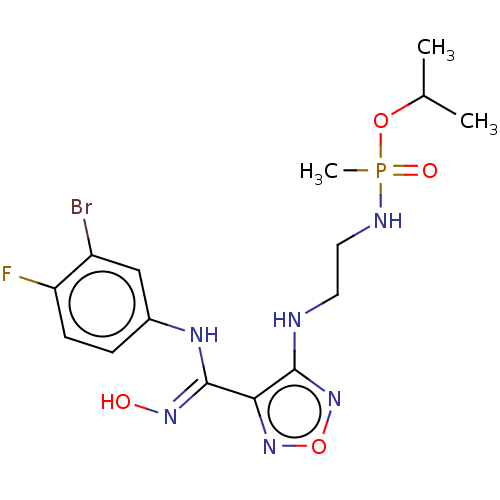
(CHEMBL4514159)Show SMILES CC(C)OP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C15H21BrFN6O4P/c1-9(2)26-28(3,25)19-7-6-18-14-13(22-27-23-14)15(21-24)20-10-4-5-12(17)11(16)8-10/h4-5,8-9,24H,6-7H2,1-3H3,(H,18,23)(H,19,25)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528770
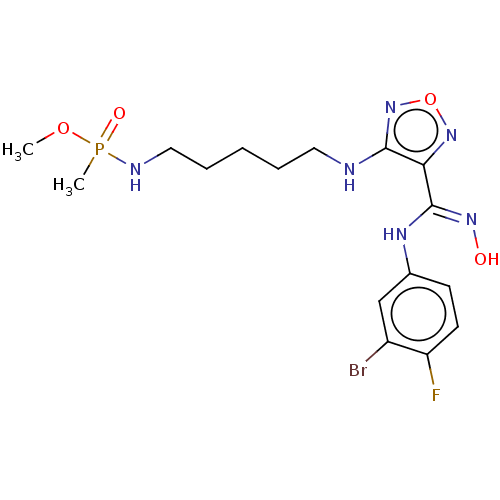
(CHEMBL4567170)Show SMILES COP(C)(=O)NCCCCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C16H23BrFN6O4P/c1-27-29(2,26)20-9-5-3-4-8-19-15-14(23-28-24-15)16(22-25)21-11-6-7-13(18)12(17)10-11/h6-7,10,25H,3-5,8-9H2,1-2H3,(H,19,24)(H,20,26)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528788

(CHEMBL4464324)Show SMILES COP(C)(=O)NCCCNc1nonc1\C(Nc1ccc(F)c(Cl)c1)=N\O Show InChI InChI=1S/C14H19ClFN6O4P/c1-25-27(2,24)18-7-3-6-17-13-12(21-26-22-13)14(20-23)19-9-4-5-11(16)10(15)8-9/h4-5,8,23H,3,6-7H2,1-2H3,(H,17,22)(H,18,24)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528778

(CHEMBL4456809)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1cccc(c1)C#N)=N\O Show InChI InChI=1S/C14H18N7O4P/c1-24-26(2,23)17-7-6-16-13-12(20-25-21-13)14(19-22)18-11-5-3-4-10(8-11)9-15/h3-5,8,22H,6-7H2,1-2H3,(H,16,21)(H,17,23)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528764
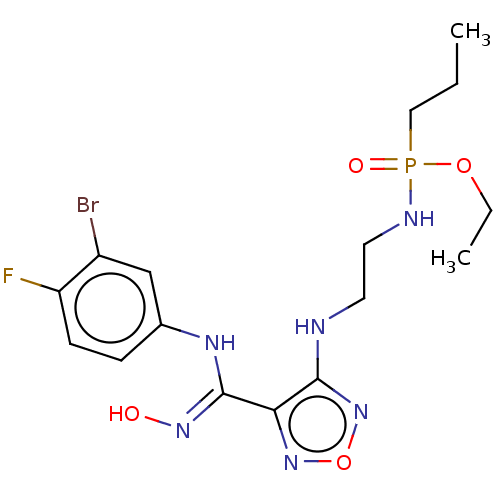
(CHEMBL4440145)Show SMILES CCCP(=O)(NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O)OCC Show InChI InChI=1S/C16H23BrFN6O4P/c1-3-9-29(26,27-4-2)20-8-7-19-15-14(23-28-24-15)16(22-25)21-11-5-6-13(18)12(17)10-11/h5-6,10,25H,3-4,7-9H2,1-2H3,(H,19,24)(H,20,26)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528759

(CHEMBL4462873)Show SMILES CCOP(=O)(CC)NCCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C16H23BrFN6O4P/c1-3-27-29(26,4-2)20-9-5-8-19-15-14(23-28-24-15)16(22-25)21-11-6-7-13(18)12(17)10-11/h6-7,10,25H,3-5,8-9H2,1-2H3,(H,19,24)(H,20,26)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528763

(CHEMBL4472697)Show SMILES CCOP(=O)(CC)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C15H21BrFN6O4P/c1-3-26-28(25,4-2)19-8-7-18-14-13(22-27-23-14)15(21-24)20-10-5-6-12(17)11(16)9-10/h5-6,9,24H,3-4,7-8H2,1-2H3,(H,18,23)(H,19,25)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528791

(CHEMBL4463815)Show InChI InChI=1S/C14H21N6O4P/c1-10-5-4-6-11(9-10)17-14(18-21)12-13(20-24-19-12)15-7-8-16-25(3,22)23-2/h4-6,9,21H,7-8H2,1-3H3,(H,15,20)(H,16,22)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528782

(CHEMBL4454093)Show SMILES COP(C)(=O)NCCCNc1nonc1\C(Nc1cccc(Br)c1)=N\O Show InChI InChI=1S/C14H20BrN6O4P/c1-24-26(2,23)17-8-4-7-16-13-12(20-25-21-13)14(19-22)18-11-6-3-5-10(15)9-11/h3,5-6,9,22H,4,7-8H2,1-2H3,(H,16,21)(H,17,23)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528777

(CHEMBL4455008)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(c1)C#N)=N\O Show InChI InChI=1S/C14H17FN7O4P/c1-25-27(2,24)18-6-5-17-13-12(21-26-22-13)14(20-23)19-10-3-4-11(15)9(7-10)8-16/h3-4,7,23H,5-6H2,1-2H3,(H,17,22)(H,18,24)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528761

(CHEMBL4552082)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(c1)C(F)(F)F)=N\O Show InChI InChI=1S/C14H17F4N6O4P/c1-27-29(2,26)20-6-5-19-12-11(23-28-24-12)13(22-25)21-8-3-4-10(15)9(7-8)14(16,17)18/h3-4,7,25H,5-6H2,1-2H3,(H,19,24)(H,20,26)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528779

(CHEMBL4514196)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C13H17BrFN6O4P/c1-24-26(2,23)17-6-5-16-12-11(20-25-21-12)13(19-22)18-8-3-4-10(15)9(14)7-8/h3-4,7,22H,5-6H2,1-2H3,(H,16,21)(H,17,23)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528781
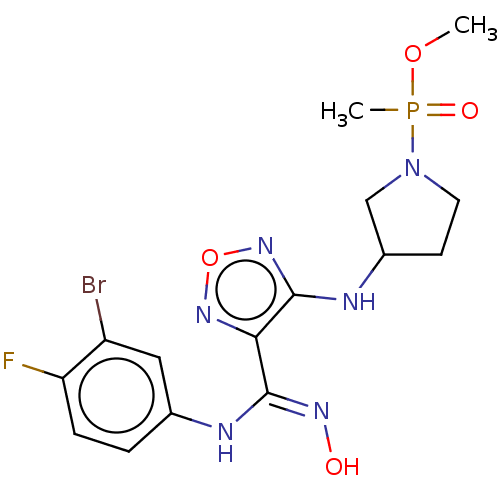
(CHEMBL4460529)Show SMILES COP(C)(=O)N1CCC(C1)Nc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C15H19BrFN6O4P/c1-26-28(2,25)23-6-5-10(8-23)19-15-13(21-27-22-15)14(20-24)18-9-3-4-12(17)11(16)7-9/h3-4,7,10,24H,5-6,8H2,1-2H3,(H,18,20)(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528762

(CHEMBL4581189)Show SMILES CCOP(=O)(CC)NCCNc1nonc1\C(Nc1cccc(Br)c1)=N\O Show InChI InChI=1S/C15H22BrN6O4P/c1-3-25-27(24,4-2)18-9-8-17-14-13(21-26-22-14)15(20-23)19-12-7-5-6-11(16)10-12/h5-7,10,23H,3-4,8-9H2,1-2H3,(H,17,22)(H,18,24)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528775

(CHEMBL4552649)Show InChI InChI=1S/C13H18BrN6O4P/c1-23-25(2,22)16-7-6-15-12-11(19-24-20-12)13(18-21)17-10-5-3-4-9(14)8-10/h3-5,8,21H,6-7H2,1-2H3,(H,15,20)(H,16,22)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528770

(CHEMBL4567170)Show SMILES COP(C)(=O)NCCCCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C16H23BrFN6O4P/c1-27-29(2,26)20-9-5-3-4-8-19-15-14(23-28-24-15)16(22-25)21-11-6-7-13(18)12(17)10-11/h6-7,10,25H,3-5,8-9H2,1-2H3,(H,19,24)(H,20,26)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528790

(CHEMBL4455935)Show SMILES CCOP(=O)(Cc1ccccc1)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C20H23BrFN6O4P/c1-2-31-33(30,13-14-6-4-3-5-7-14)24-11-10-23-19-18(27-32-28-19)20(26-29)25-15-8-9-17(22)16(21)12-15/h3-9,12,29H,2,10-11,13H2,1H3,(H,23,28)(H,24,30)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528760

(CHEMBL4444787)Show SMILES CCOP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C14H19BrFN6O4P/c1-3-25-27(2,24)18-7-6-17-13-12(21-26-22-13)14(20-23)19-9-4-5-11(16)10(15)8-9/h4-5,8,23H,3,6-7H2,1-2H3,(H,17,22)(H,18,24)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528785

(CHEMBL4514159)Show SMILES CC(C)OP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C15H21BrFN6O4P/c1-9(2)26-28(3,25)19-7-6-18-14-13(22-27-23-14)15(21-24)20-10-4-5-12(17)11(16)8-10/h4-5,8-9,24H,6-7H2,1-3H3,(H,18,23)(H,19,25)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528783

(CHEMBL4455785)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1cccc(c1)C(F)(F)F)=N\O Show InChI InChI=1S/C14H18F3N6O4P/c1-26-28(2,25)19-7-6-18-12-11(22-27-23-12)13(21-24)20-10-5-3-4-9(8-10)14(15,16)17/h3-5,8,24H,6-7H2,1-2H3,(H,18,23)(H,19,25)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528780

(CHEMBL4436582)Show SMILES COP(C)(=O)NCCCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C15H21BrFN6O4P/c1-26-28(2,25)19-8-4-3-7-18-14-13(22-27-23-14)15(21-24)20-10-5-6-12(17)11(16)9-10/h5-6,9,24H,3-4,7-8H2,1-2H3,(H,18,23)(H,19,25)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528769

(CHEMBL4439914)Show SMILES CCOP(=O)(Cc1cccc2ccccc12)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C24H25BrFN6O4P/c1-2-35-37(34,15-17-8-5-7-16-6-3-4-9-19(16)17)28-13-12-27-23-22(31-36-32-23)24(30-33)29-18-10-11-21(26)20(25)14-18/h3-11,14,33H,2,12-13,15H2,1H3,(H,27,32)(H,28,34)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528781

(CHEMBL4460529)Show SMILES COP(C)(=O)N1CCC(C1)Nc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C15H19BrFN6O4P/c1-26-28(2,25)23-6-5-10(8-23)19-15-13(21-27-22-15)14(20-24)18-9-3-4-12(17)11(16)7-9/h3-4,7,10,24H,5-6,8H2,1-2H3,(H,18,20)(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged human IDO1 expressed in Escherichia coli assessed as reduction in N-formylkynurenine formation using ... |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528787

(CHEMBL4441056)Show SMILES CCOP(=O)(NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O)c1ccccc1 Show InChI InChI=1S/C19H21BrFN6O4P/c1-2-30-32(29,14-6-4-3-5-7-14)23-11-10-22-18-17(26-31-27-18)19(25-28)24-13-8-9-16(21)15(20)12-13/h3-9,12,28H,2,10-11H2,1H3,(H,22,27)(H,23,29)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528765

(CHEMBL4565478)Show SMILES CCCP(=O)(NCCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O)OCC Show InChI InChI=1S/C17H25BrFN6O4P/c1-3-10-30(27,28-4-2)21-9-5-8-20-16-15(24-29-25-16)17(23-26)22-12-6-7-14(19)13(18)11-12/h6-7,11,26H,3-5,8-10H2,1-2H3,(H,20,25)(H,21,27)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528768
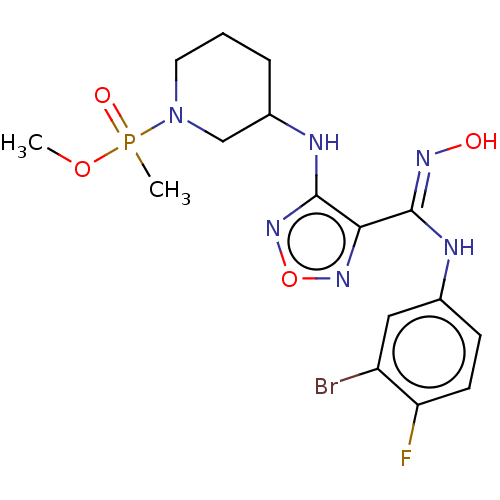
(CHEMBL4464199)Show SMILES COP(C)(=O)N1CCCC(C1)Nc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C16H21BrFN6O4P/c1-27-29(2,26)24-7-3-4-11(9-24)20-16-14(22-28-23-16)15(21-25)19-10-5-6-13(18)12(17)8-10/h5-6,8,11,25H,3-4,7,9H2,1-2H3,(H,19,21)(H,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528786

(CHEMBL4573286)Show SMILES CCOP(C)(=O)N1CCC(CC1)Nc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C17H23BrFN6O4P/c1-3-28-30(2,27)25-8-6-11(7-9-25)20-17-15(23-29-24-17)16(22-26)21-12-4-5-14(19)13(18)10-12/h4-5,10-11,26H,3,6-9H2,1-2H3,(H,20,24)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528766

(CHEMBL4543296)Show SMILES CCOP(=O)(CC)N1CCC(CC1)Nc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C18H25BrFN6O4P/c1-3-29-31(28,4-2)26-9-7-12(8-10-26)21-18-16(24-30-25-18)17(23-27)22-13-5-6-15(20)14(19)11-13/h5-6,11-12,27H,3-4,7-10H2,1-2H3,(H,21,25)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528767
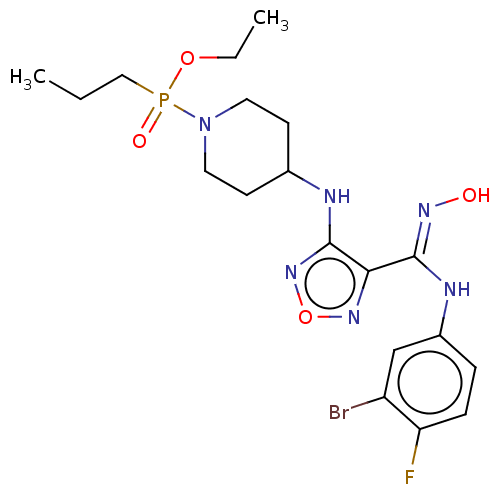
(CHEMBL4540060)Show SMILES CCCP(=O)(OCC)N1CCC(CC1)Nc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C19H27BrFN6O4P/c1-3-11-32(29,30-4-2)27-9-7-13(8-10-27)22-19-17(25-31-26-19)18(24-28)23-14-5-6-16(21)15(20)12-14/h5-6,12-13,28H,3-4,7-11H2,1-2H3,(H,22,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 2
(Homo sapiens (Human)) | BDBM50528763

(CHEMBL4472697)Show SMILES CCOP(=O)(CC)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C15H21BrFN6O4P/c1-3-26-28(25,4-2)19-8-7-18-14-13(22-27-23-14)15(21-24)20-10-5-6-12(17)11(16)9-10/h5-6,9,24H,3-4,7-8H2,1-2H3,(H,18,23)(H,19,25)(H,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human IDO2 expressed in Escherichia coli expression system |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 2
(Homo sapiens (Human)) | BDBM50528779

(CHEMBL4514196)Show SMILES COP(C)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C13H17BrFN6O4P/c1-24-26(2,23)17-6-5-16-12-11(20-25-21-12)13(19-22)18-8-3-4-10(15)9(14)7-8/h3-4,7,22H,5-6H2,1-2H3,(H,16,21)(H,17,23)(H,18,19) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human IDO2 expressed in Escherichia coli expression system |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 2
(Homo sapiens (Human)) | BDBM50528780

(CHEMBL4436582)Show SMILES COP(C)(=O)NCCCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C15H21BrFN6O4P/c1-26-28(2,25)19-8-4-3-7-18-14-13(22-27-23-14)15(21-24)20-10-5-6-12(17)11(16)9-10/h5-6,9,24H,3-4,7-8H2,1-2H3,(H,18,23)(H,19,25)(H,20,21) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human IDO2 expressed in Escherichia coli expression system |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 2
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human IDO2 expressed in Escherichia coli expression system |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528776
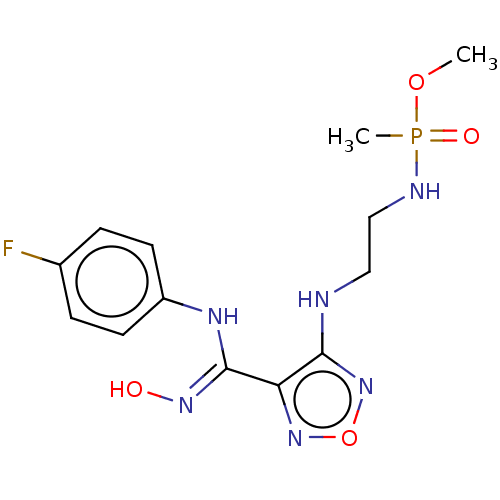
(CHEMBL4519220)Show InChI InChI=1S/C13H18FN6O4P/c1-23-25(2,22)16-8-7-15-12-11(19-24-20-12)13(18-21)17-10-5-3-9(14)4-6-10/h3-6,21H,7-8H2,1-2H3,(H,15,20)(H,16,22)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50528772

(CHEMBL4556184)Show SMILES COP(C)(=O)NC1CCCN(C1)c1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C16H21BrFN6O4P/c1-27-29(2,26)23-11-4-3-7-24(9-11)16-14(21-28-22-16)15(20-25)19-10-5-6-13(18)12(17)8-10/h5-6,8,11,25H,3-4,7,9H2,1-2H3,(H,19,20)(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate incubated for 48 hrs |
Eur J Med Chem 182: (2019)
Article DOI: 10.1016/j.ejmech.2019.111629
BindingDB Entry DOI: 10.7270/Q2BZ69GN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data