

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555731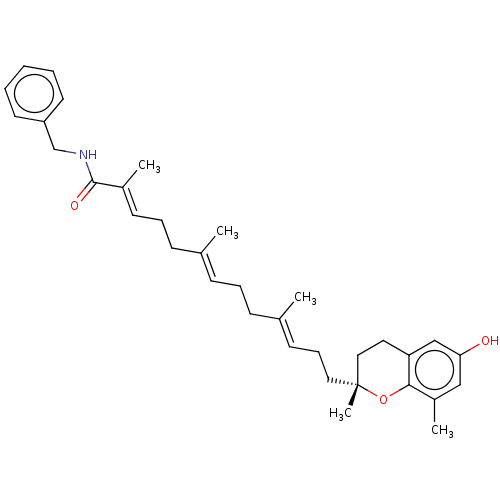 (CHEMBL4743283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50527521 (CHEMBL4463788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555732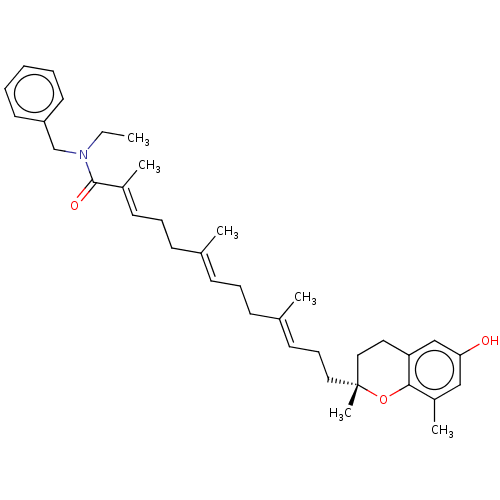 (CHEMBL4757880) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555734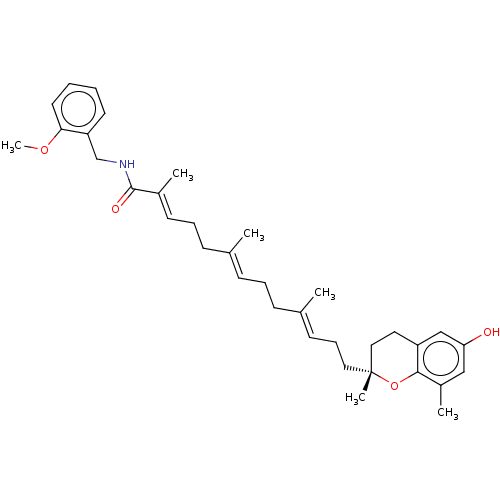 (CHEMBL4746603) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555733 (CHEMBL4739891) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555751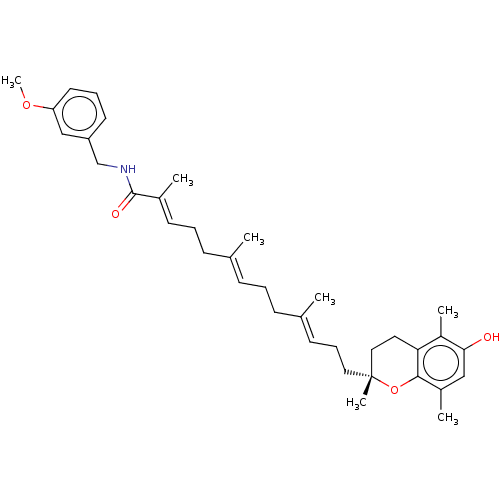 (CHEMBL4779142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555736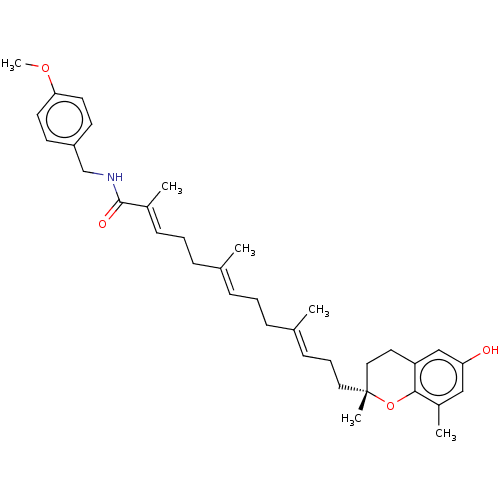 (CHEMBL4743535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555752 (CHEMBL4745718) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555735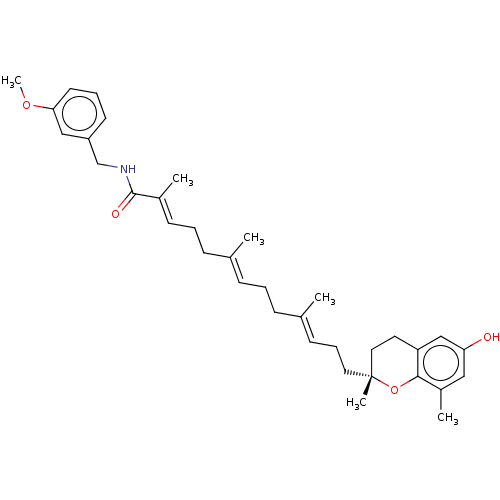 (CHEMBL4751589) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555750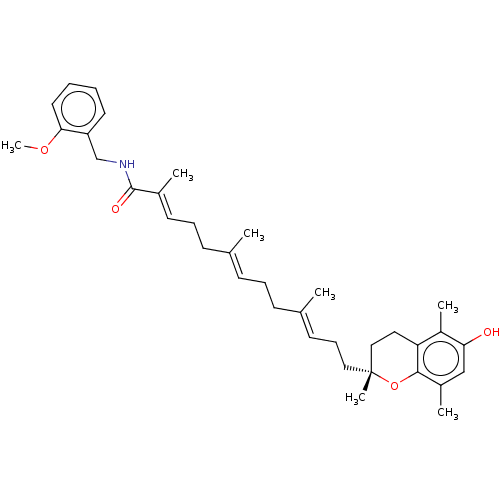 (CHEMBL4789195) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555738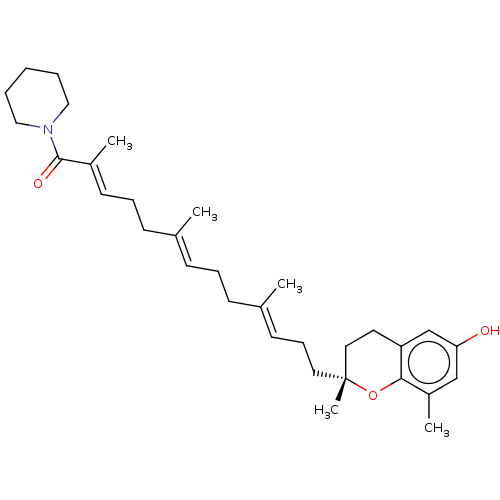 (CHEMBL4789900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555737 (CHEMBL4754092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555730 (CHEMBL4747347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555748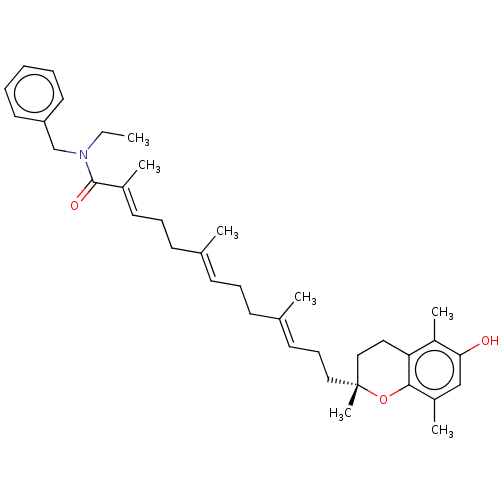 (CHEMBL4753605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555739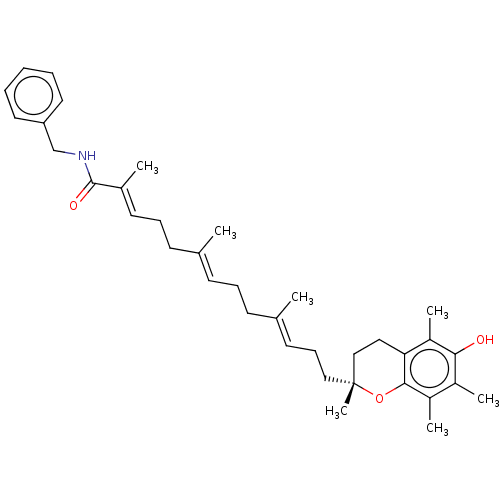 (CHEMBL4786973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555747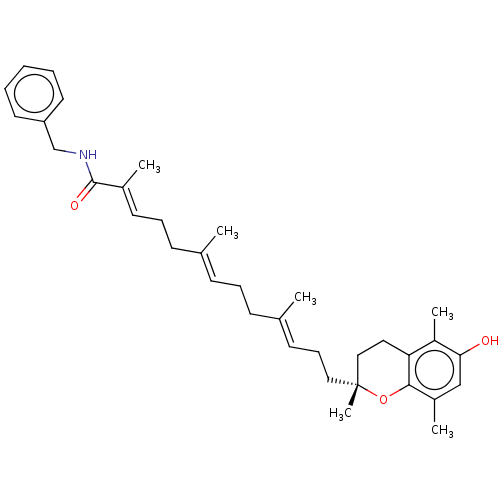 (CHEMBL4782989) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555736 (CHEMBL4743535) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555745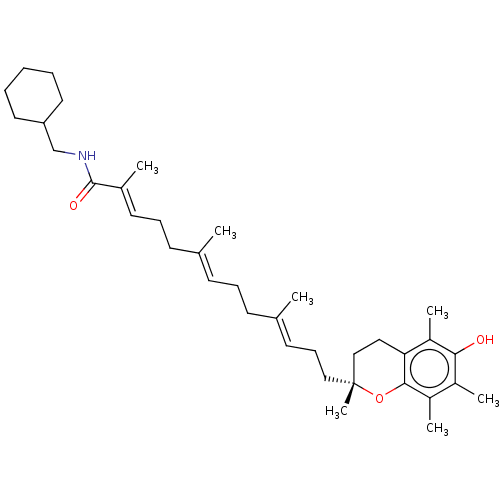 (CHEMBL4757548) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555741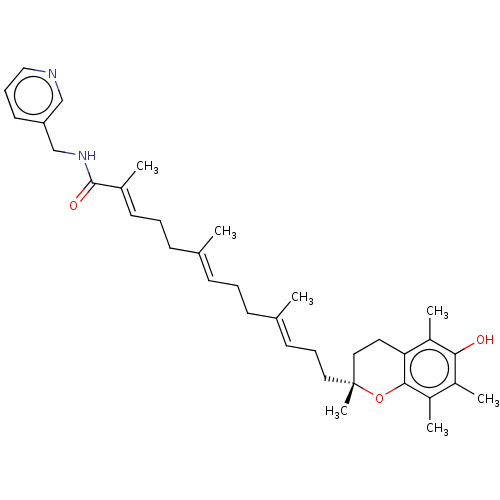 (CHEMBL4758409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555744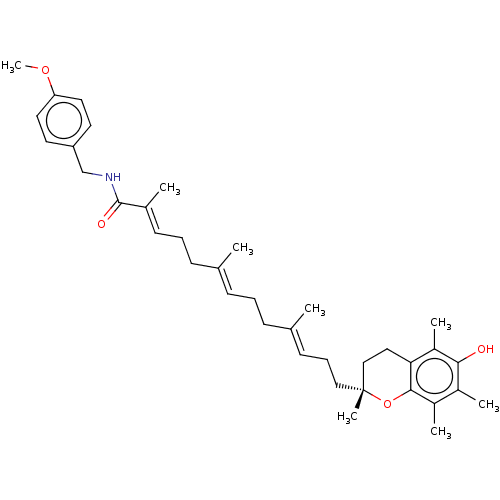 (CHEMBL4746263) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555753 (CHEMBL4747644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555731 (CHEMBL4743283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555730 (CHEMBL4747347) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555745 (CHEMBL4757548) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50527521 (CHEMBL4463788) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 345 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555749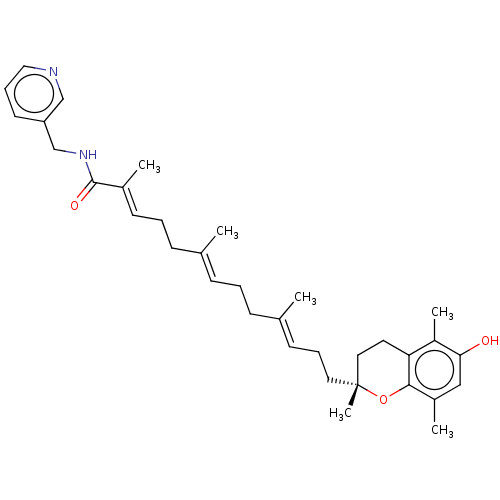 (CHEMBL4792006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 362 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555749 (CHEMBL4792006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 401 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555729 (CHEMBL4761835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 401 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555733 (CHEMBL4739891) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 415 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555742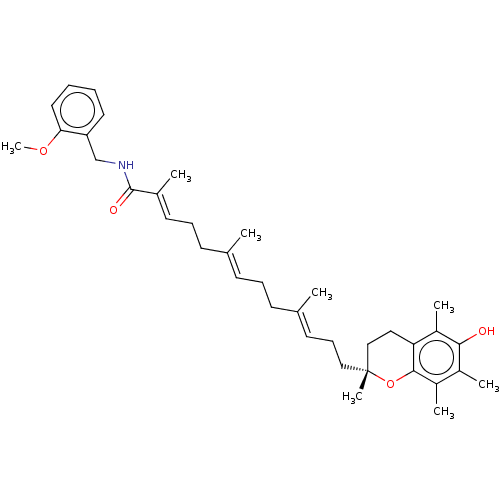 (CHEMBL4779134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555748 (CHEMBL4753605) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 432 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 521 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555732 (CHEMBL4757880) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 525 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555747 (CHEMBL4782989) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 538 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555737 (CHEMBL4754092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 561 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555741 (CHEMBL4758409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 563 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555744 (CHEMBL4746263) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 572 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555739 (CHEMBL4786973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 595 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555740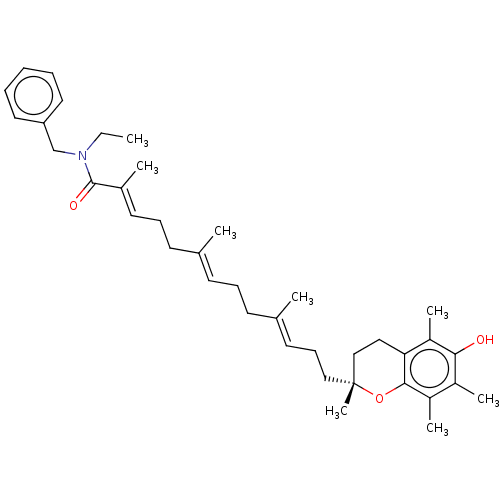 (CHEMBL4745334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 623 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555752 (CHEMBL4745718) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 724 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555743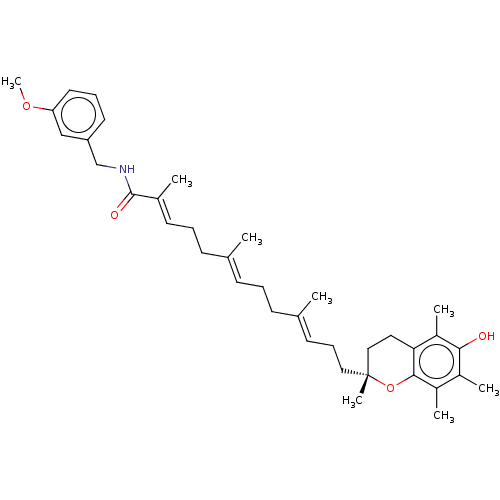 (CHEMBL4782136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 725 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000541 ((+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555743 (CHEMBL4782136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 839 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555751 (CHEMBL4779142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 997 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555746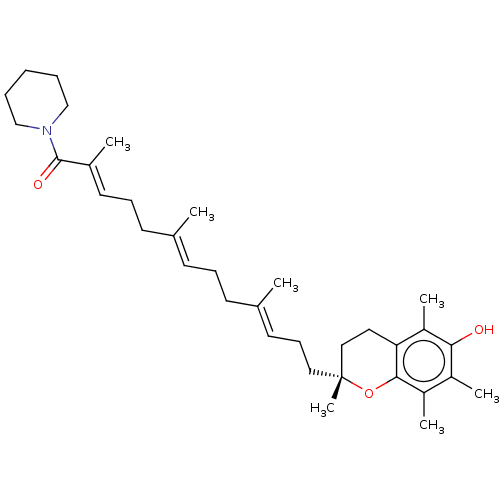 (CHEMBL4763043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 998 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555754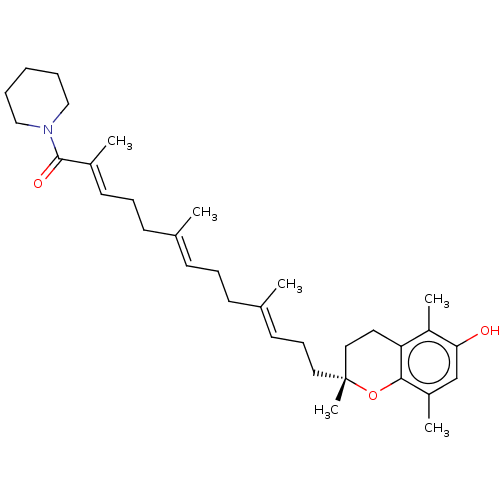 (CHEMBL4754425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human 5-LO expressed in Escherichia coli BL21(DE3) assessed as reduction in LTB4 and 5-H(P)ETE formation using arachidonic ... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555753 (CHEMBL4747644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555742 (CHEMBL4779134) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555729 (CHEMBL4761835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50555734 (CHEMBL4746603) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 5-LO in human PMNL cells assessed as reduction in LTB4, 5-H(P)ETE, 12-HETE and 15-HETE products formation using arachidonic acid as sub... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112518 BindingDB Entry DOI: 10.7270/Q21J9FF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |