

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM50558002 (CHEMBL4777271) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558013 (CHEMBL4755831) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558015 (CHEMBL4745681) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558012 (CHEMBL4760286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558016 (CHEMBL4778401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558005 (CHEMBL4780337) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558009 (CHEMBL4753594) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558014 (CHEMBL4750835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558003 (CHEMBL4797578) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558017 (CHEMBL4761359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 439 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558010 (CHEMBL4764457) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558006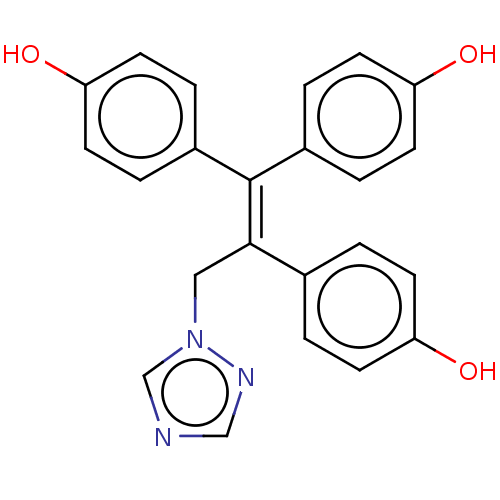 (CHEMBL4753984) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558007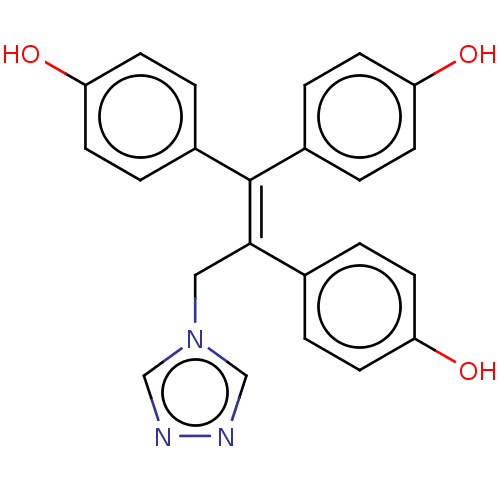 (CHEMBL4749692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558001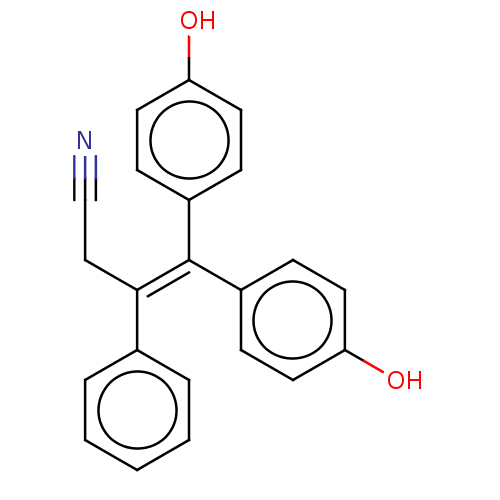 (CHEMBL4763409) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558011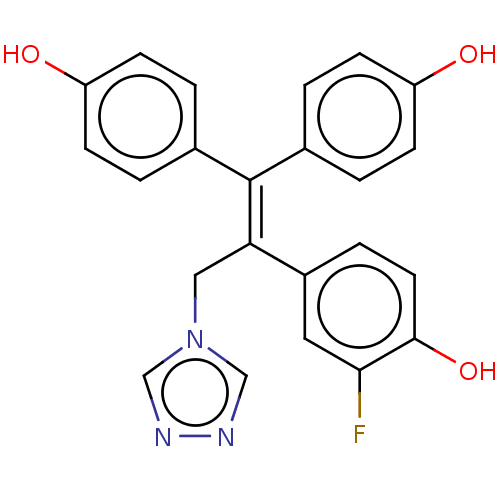 (CHEMBL4752568) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558004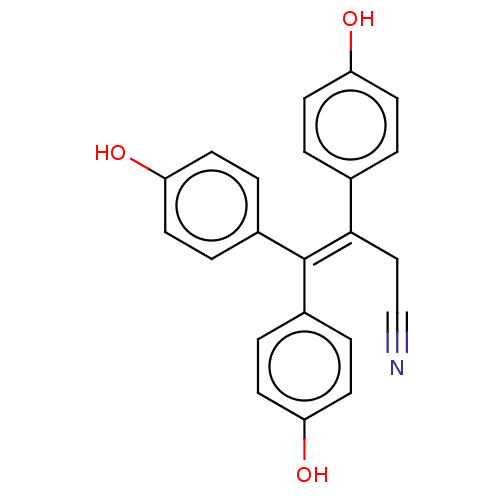 (CHEMBL4747849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50558008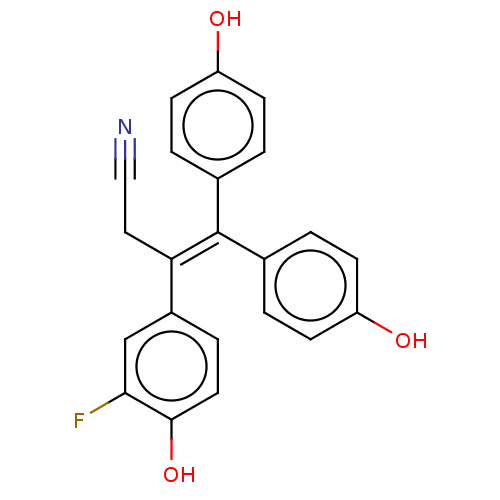 (CHEMBL4743255) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50121317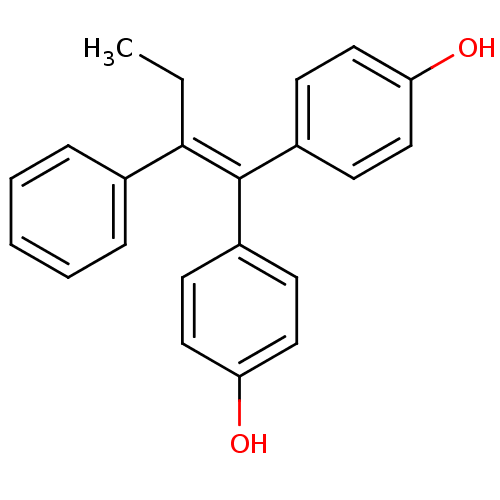 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human aromatase incubated for 30 mins using fluorometric substrate 7-methoxy-4-trifluoromethylcoumarin in presence of NADPH... | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50558002 (CHEMBL4777271) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of ES2 from recombinant human ER-alpha incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558013 (CHEMBL4755831) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 296 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of ES2 from recombinant human ER-beta incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558007 (CHEMBL4749692) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of ES2 from recombinant human ER-beta incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558009 (CHEMBL4753594) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 56 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of ES2 from recombinant human ER-beta incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558005 (CHEMBL4780337) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of ES2 from recombinant human ER-beta incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50558002 (CHEMBL4777271) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 41 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of ES2 from recombinant human ER-beta incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50558007 (CHEMBL4749692) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 943 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of ES2 from recombinant human ER-alpha incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50558009 (CHEMBL4753594) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 85 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of ES2 from recombinant human ER-alpha incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50558013 (CHEMBL4755831) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of ES2 from recombinant human ER-alpha incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50121317 (1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 307 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of ES2 from recombinant human ER-beta incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50558005 (CHEMBL4780337) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 98 | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of ES2 from recombinant human ER-alpha incubated for 2 hrs by fluorescence based assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.5b01677 BindingDB Entry DOI: 10.7270/Q2B2800F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||