

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50124048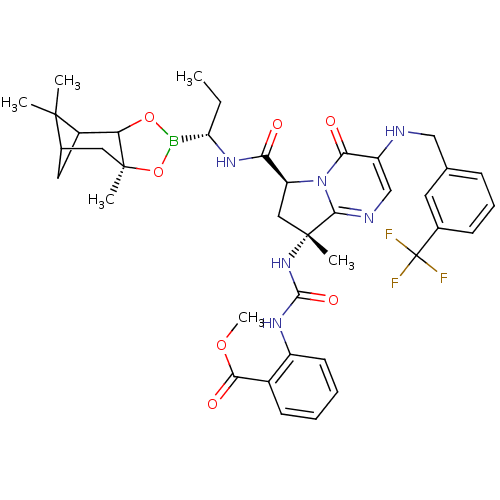 (2-((R)-3-{(S)-8-Methyl-4-oxo-3-(3-trifluoromethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124044 ((S)-8-Methyl-8-[(3-methylsulfanyl-phenylcarbamoyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124056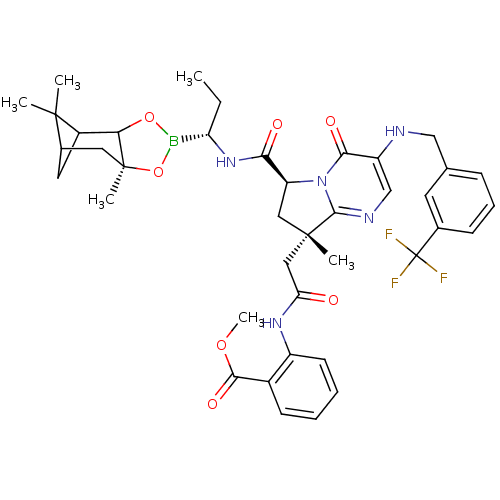 (2-((S)-2-{(S)-8-Methyl-4-oxo-3-(3-trifluoromethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124063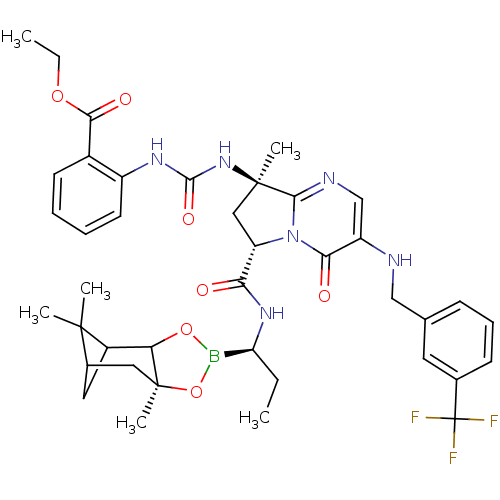 (2-((R)-3-{(S)-8-Methyl-4-oxo-3-(3-trifluoromethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124055 ((S)-8-Methyl-8-[(R)-3-(3-methylsulfanyl-phenyl)-ur...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124049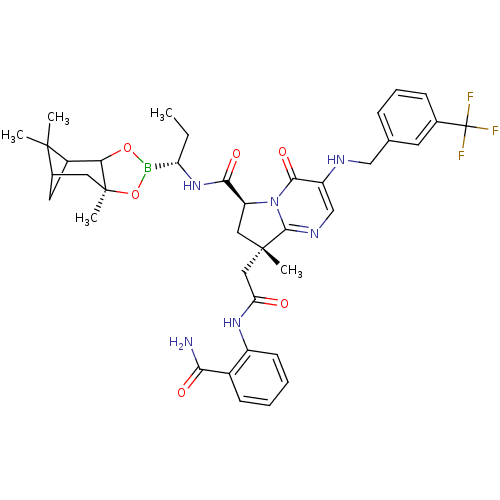 ((S)-8-[(2-Carbamoyl-phenylcarbamoyl)-methyl]-8-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124039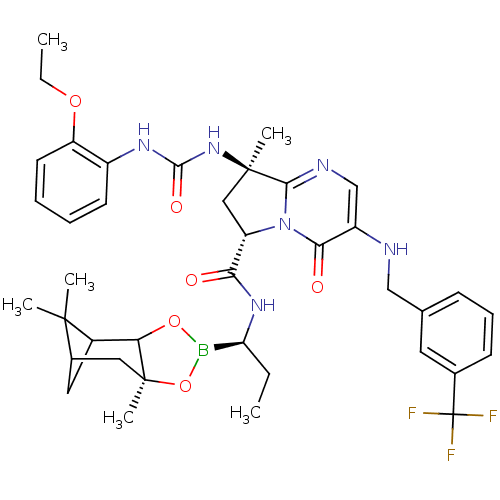 ((S)-8-[(R)-3-(2-Ethoxy-phenyl)-ureido]-8-methyl-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124035 ((S)-8-Methyl-4-oxo-8-((S)-phenylcarbamoylmethyl)-3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124042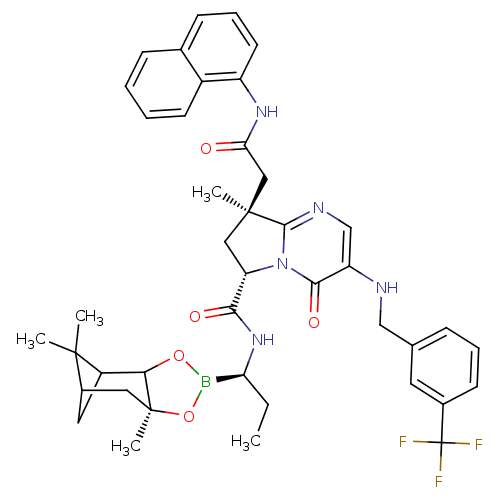 ((S)-8-Methyl-8-((S)-naphthalen-1-ylcarbamoylmethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124057 ((S)-8-Methyl-8-[(R)-3-(2-nitro-phenyl)-ureido]-4-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124034 ((S)-8-Methyl-8-[(2-methylcarbamoyl-phenylcarbamoyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124038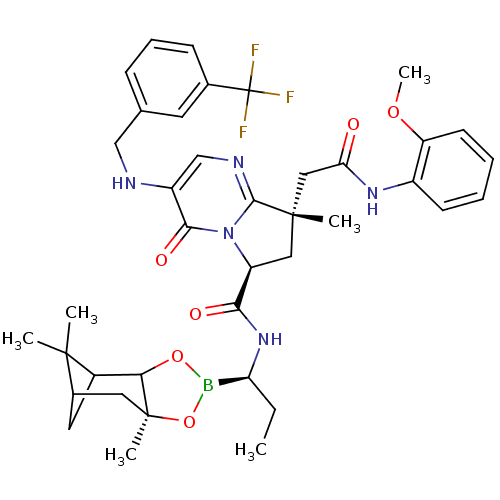 ((S)-8-[(2-Methoxy-phenylcarbamoyl)-methyl]-8-methy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124053 ((S)-8-Methyl-8-((R)-3-naphthalen-1-yl-ureido)-4-ox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124043 ((S)-8-Methyl-4-oxo-8-[(R)-3-(2-phenoxy-phenyl)-ure...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124036 ((S)-8-[(R)-3-(2-Methoxy-phenyl)-ureido]-8-methyl-4...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124040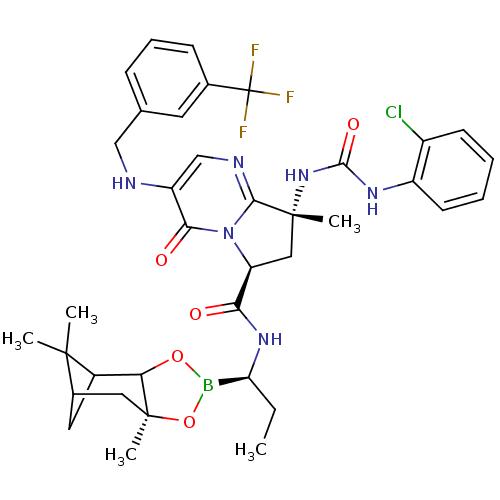 ((S)-8-[(R)-3-(2-Chloro-phenyl)-ureido]-8-methyl-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124033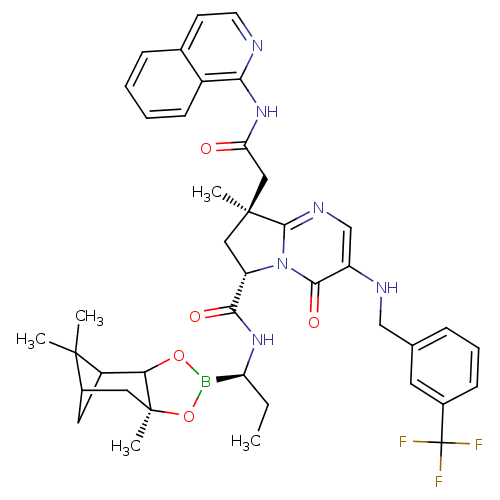 ((S)-8-((S)-Isoquinolin-1-ylcarbamoylmethyl)-8-meth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124062 ((S)-8-[(R)-3-(3-Fluoro-phenyl)-ureido]-8-methyl-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124066 ((S)-8-Methyl-8-[(R)-3-(2-methylsulfanyl-phenyl)-ur...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124046 ((S)-8-Methyl-4-oxo-8-((R)-3-o-tolyl-ureido)-3-(3-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124052 ((S)-8-Methyl-4-oxo-8-((R)-3-phenyl-ureido)-3-(3-tr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124058 ((S)-8-[(R)-3-(4-Fluoro-phenyl)-ureido]-8-methyl-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124067 (4-((R)-3-{(S)-8-Methyl-4-oxo-3-(3-trifluoromethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124069 ((S)-8-[(R)-3-(4-Chloro-phenyl)-ureido]-8-methyl-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124047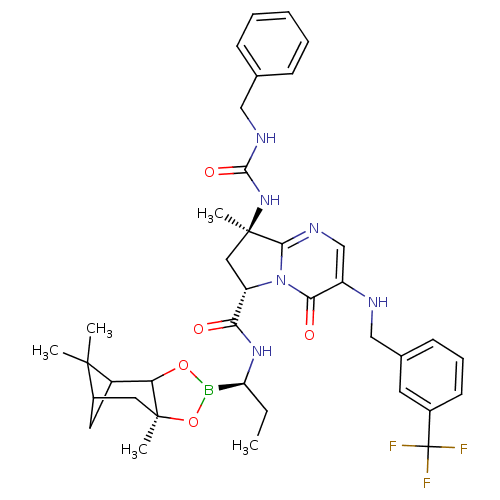 ((S)-8-((R)-3-Benzyl-ureido)-8-methyl-4-oxo-3-(3-tr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124061 (CHEMBL164441 | {(S)-8-Methyl-4-oxo-3-(3-trifluorom...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124045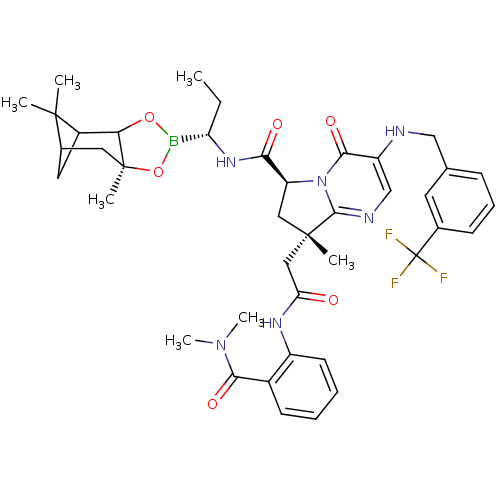 ((S)-8-Methyl-8-[(2-dimethylcarbamoyl-phenylcarbamo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124041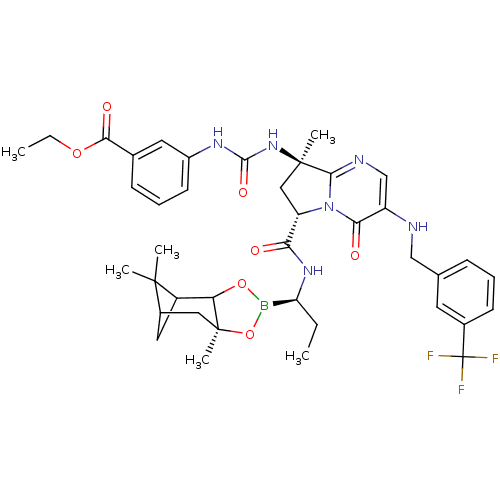 (3-((R)-3-{(S)-8-Methyl-4-oxo-3-(3-trifluoromethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124050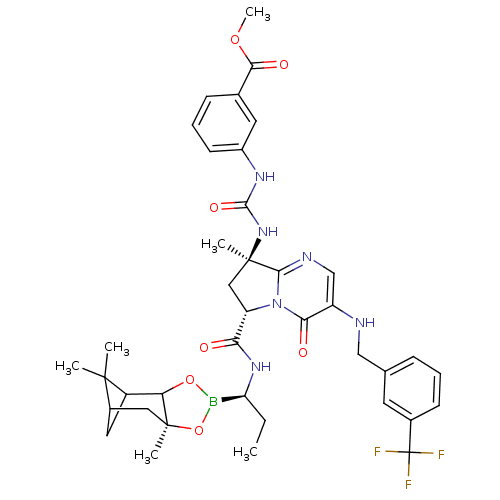 (3-((R)-3-{(S)-8-Methyl-4-oxo-3-(3-trifluoromethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124054 ((S)-8-Methyl-8-[(R)-3-(4-methylsulfanyl-phenyl)-ur...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124059 ((S)-8-Methyl-4-oxo-8-((R)-phenylacetylamino)-3-(3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124037 ((S)-8-Methyl-8-[(2-nitro-phenylcarbamoyl)-methyl]-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124051 ((S)-8-((R)-Benzoylamino)-8-methyl-4-oxo-3-(3-trifl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124068 ((S)-8-Methyl-4-oxo-8-[(R)-3-(4-phenoxy-phenyl)-ure...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124065 (2-((R)-3-{(S)-8-Methyl-4-oxo-3-(3-trifluoromethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124064 ((S)-4-Oxo-3-(3-trifluoromethyl-benzylamino)-4,6,7,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124060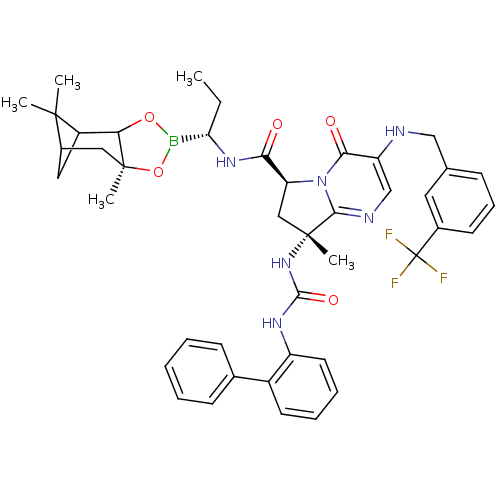 ((S)-8-((R)-3-Biphenyl-2-yl-ureido)-8-methyl-4-oxo-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||