

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50067004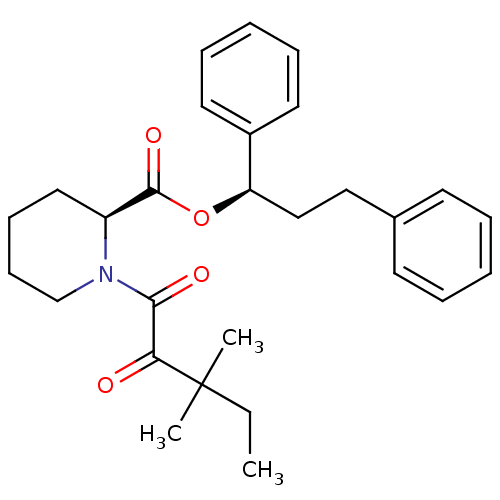 ((S)-1-(3,3-Dimethyl-2-oxo-pentanoyl)-piperidine-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human FKBP12 expressed in Escherichia coli using succinyl-Ala-Phe-Pro-Phe-4-nitroanilide as substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50335225 ((S)-1-Phenylmethanesulfonyl-piperidine-2-carboxyli...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human FKBP12 expressed in Escherichia coli using succinyl-Ala-Phe-Pro-Phe-4-nitroanilide as substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50565509 (CHEMBL4790993) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FKBP12 assessed as reduction in PPIase activity using succinyl-Ala-Leu-Pro-Phe-4-nitroanilide as substrate incubated for 4 mins b... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50565511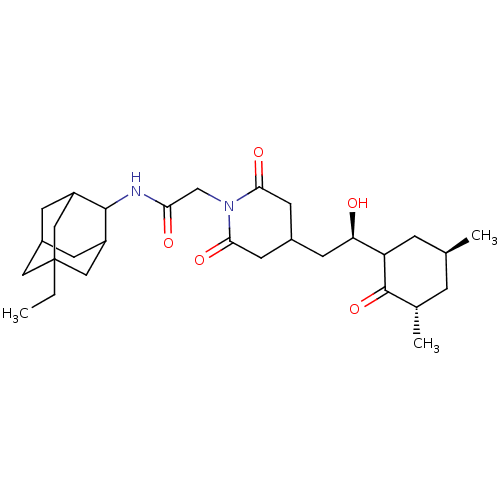 (CHEMBL4788177) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FKBP12 assessed as reduction in PPIase activity using succinyl-Ala-Leu-Pro-Phe-4-nitroanilide as substrate incubated for 4 mins b... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50565510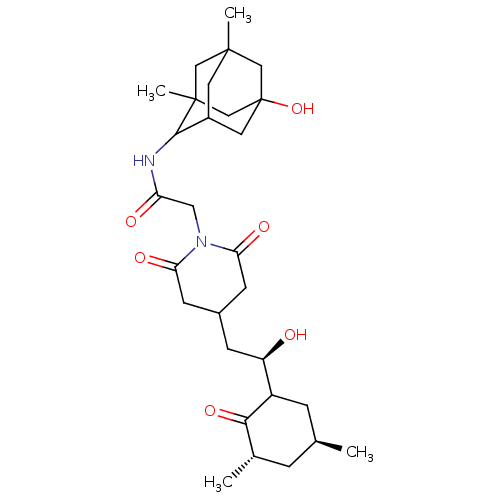 (CHEMBL4794577) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FKBP12 assessed as reduction in PPIase activity using succinyl-Ala-Leu-Pro-Phe-4-nitroanilide as substrate incubated for 4 mins b... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50565512 (CHEMBL4798300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FKBP12 assessed as reduction in PPIase activity using succinyl-Ala-Leu-Pro-Phe-4-nitroanilide as substrate incubated for 4 mins b... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM50437711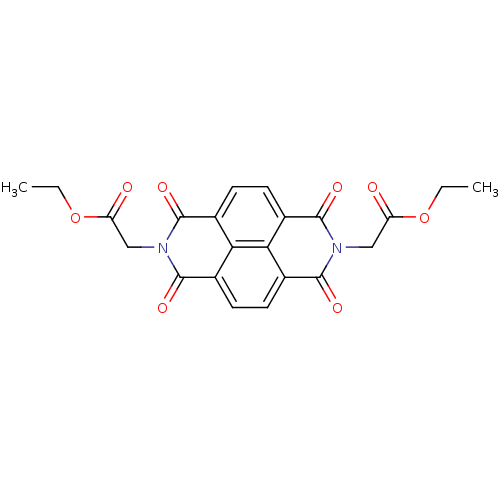 (CHEMBL2409076) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Pin1 assessed as reduction in PPIase activity using Suc-Ala-Glu-Pro-Phe-MCA as substrate measured for 20 secs by fluorescence met... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Outer membrane protein MIP (Legionella pneumophila) | BDBM50565509 (CHEMBL4790993) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Legionella pneumophila Mip assessed as reduction in PPIase activity using succinyl-Ala-Leu-Pro-Phe-4-nitroanilide as substrate incubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Outer membrane protein MIP (Legionella pneumophila) | BDBM50565510 (CHEMBL4794577) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Legionella pneumophila Mip assessed as reduction in PPIase activity using succinyl-Ala-Leu-Pro-Phe-4-nitroanilide as substrate incubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Outer membrane protein MIP (Legionella pneumophila) | BDBM50565511 (CHEMBL4788177) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Legionella pneumophila Mip assessed as reduction in PPIase activity using succinyl-Ala-Leu-Pro-Phe-4-nitroanilide as substrate incubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Outer membrane protein MIP (Legionella pneumophila) | BDBM50565512 (CHEMBL4798300) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Legionella pneumophila Mip assessed as reduction in PPIase activity using succinyl-Ala-Leu-Pro-Phe-4-nitroanilide as substrate incubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Outer membrane protein MIP (Legionella pneumophila) | BDBM50565513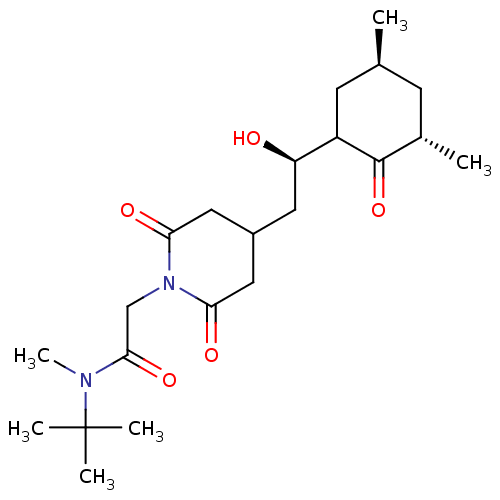 (CHEMBL4779246) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Legionella pneumophila Mip assessed as reduction in PPIase activity using succinyl-Ala-Leu-Pro-Phe-4-nitroanilide as substrate incubate... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50565513 (CHEMBL4779246) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FKBP12 assessed as reduction in PPIase activity using succinyl-Ala-Leu-Pro-Phe-4-nitroanilide as substrate incubated for 4 mins b... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Homo sapiens (Human)) | BDBM31883 (9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to Pin1 (unknown origin) preincubated for 2 hrs followed by UV irradiation for 15 mins by SDS-PAGE gel based photoaffinity labelling... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00911 BindingDB Entry DOI: 10.7270/Q22V2KW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||