

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572808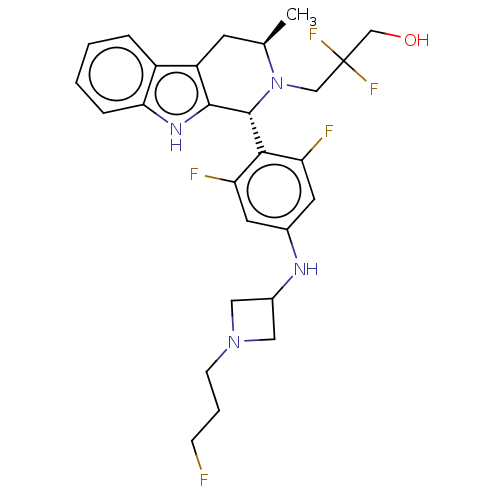 (Gdc-9545 | Giredestrant | RO-7197597 | RO7197597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572809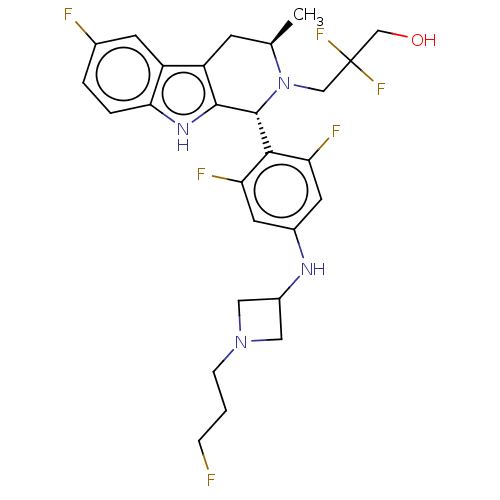 (CHEMBL4866043) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572829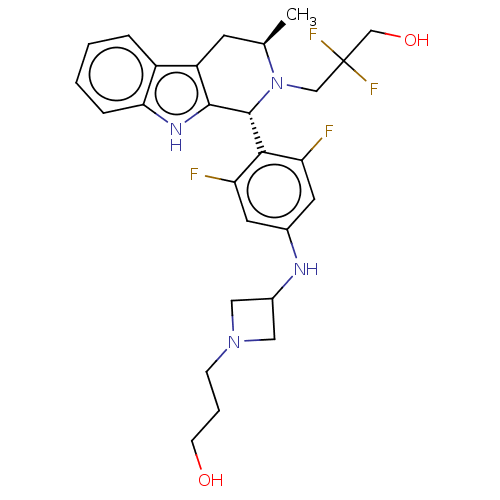 (CHEMBL4856969) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572825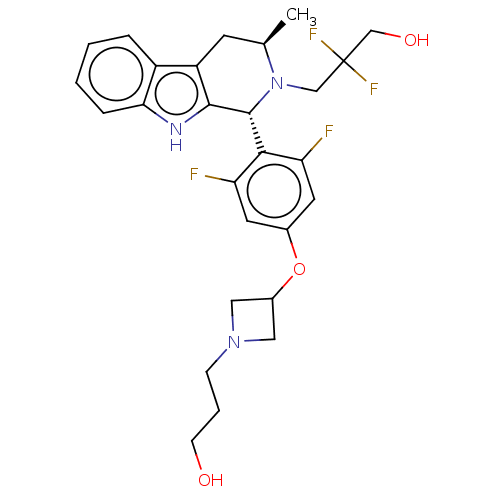 (CHEMBL4856892) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572832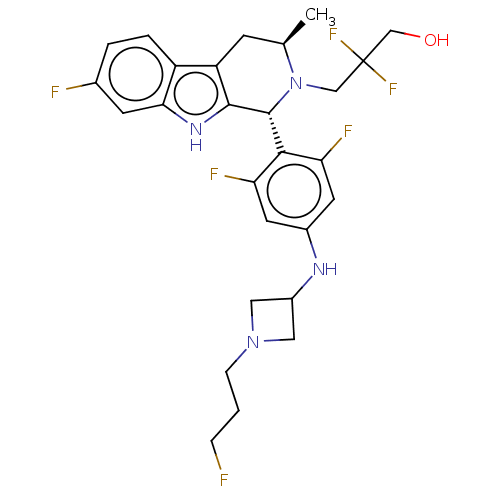 (CHEMBL4845726) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572828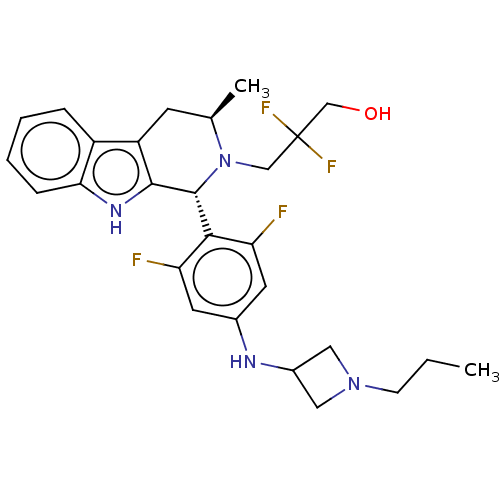 (CHEMBL4864829) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572826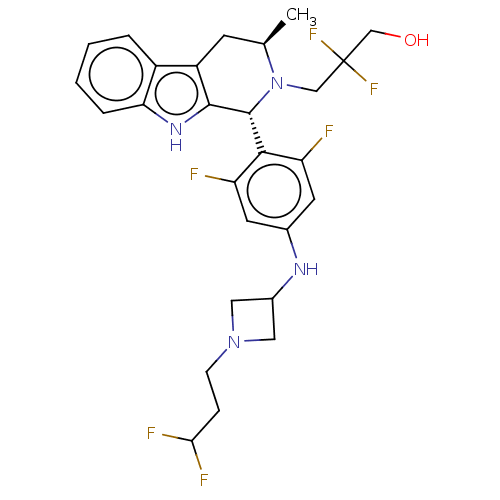 (CHEMBL4869698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM368199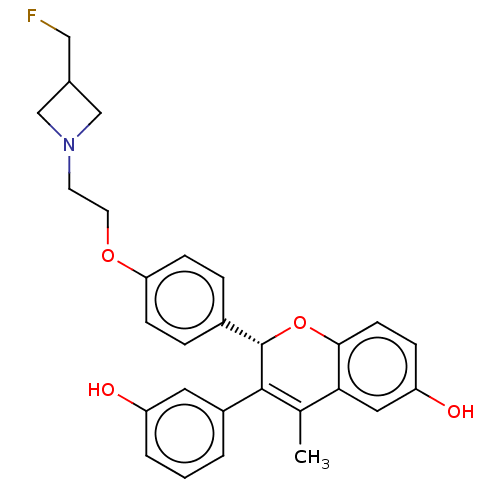 ((S)-2-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572821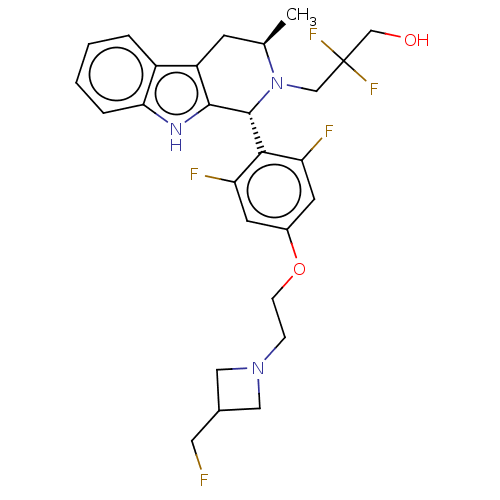 (CHEMBL4871161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572824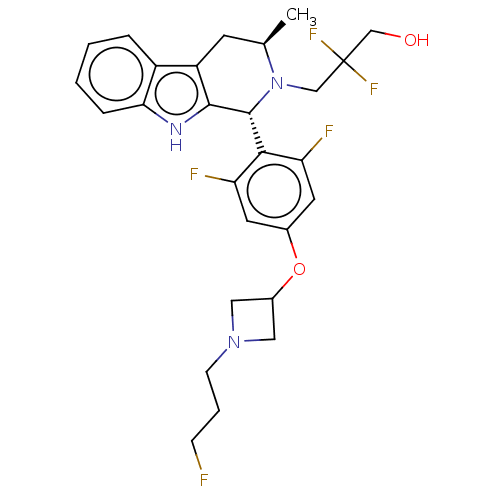 (CHEMBL4857736) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50542086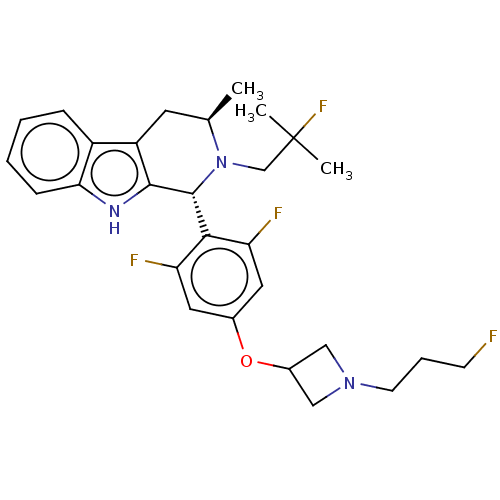 (CHEMBL4649161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572831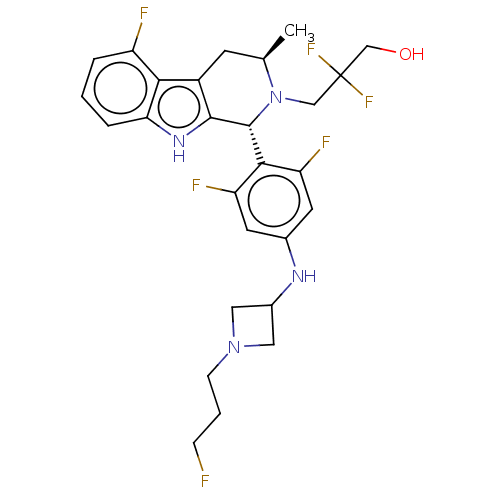 (CHEMBL4877338) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572823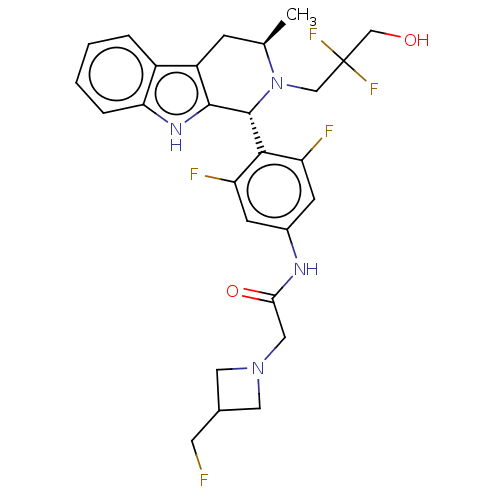 (CHEMBL4860671) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572834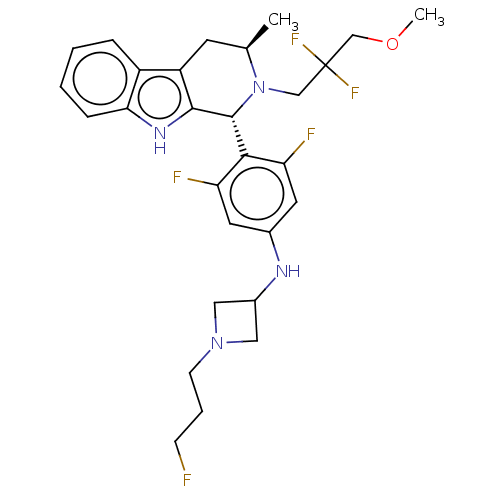 (CHEMBL4853118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM299111 (US10125135, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572822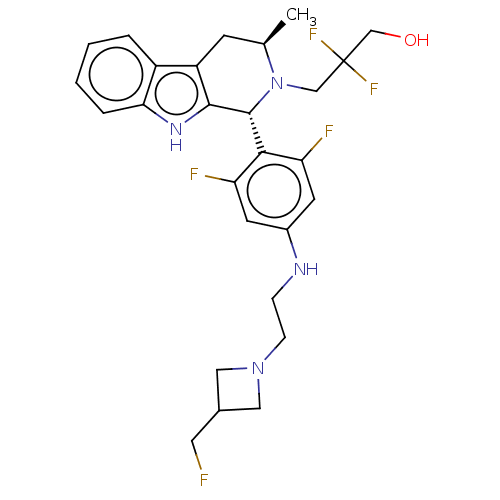 (CHEMBL4866232) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50238741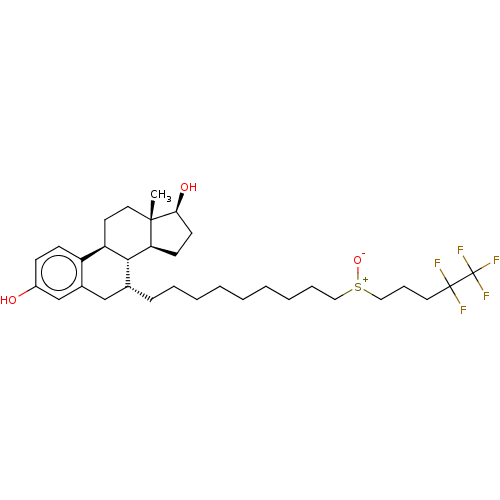 (CHEBI:31638 | Faslodex | Fulvestrant | ICI-182780 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572833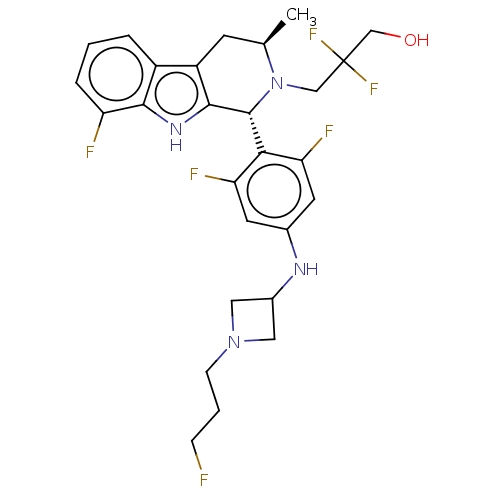 (CHEMBL4873860) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572827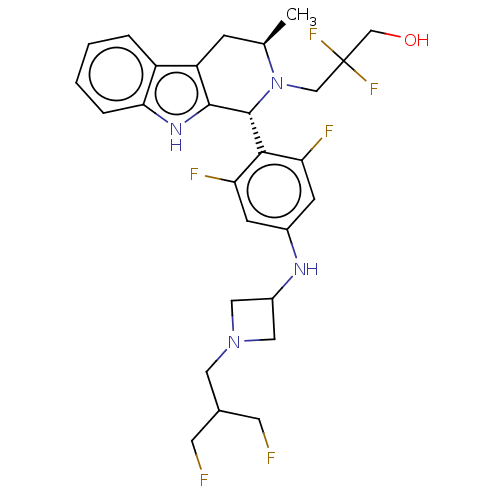 (CHEMBL4871601) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572815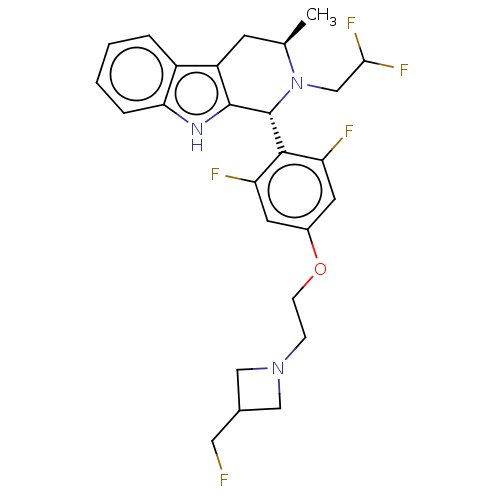 (CHEMBL4847707) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572820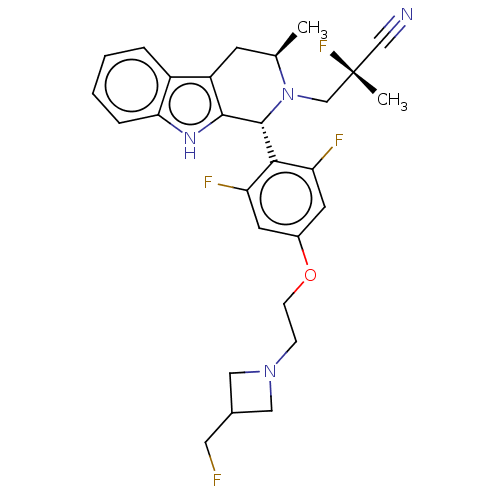 (CHEMBL4850919) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572817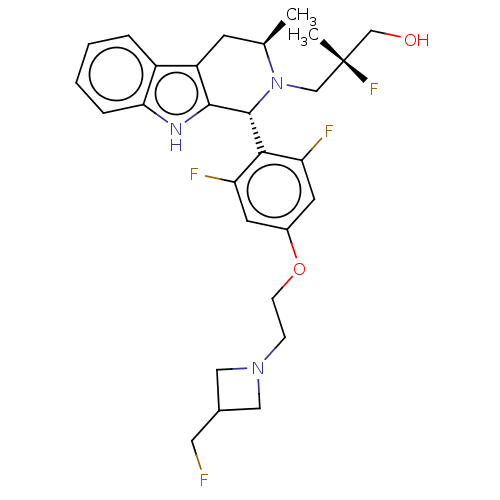 (CHEMBL4854930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572814 (CHEMBL4867106) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50503111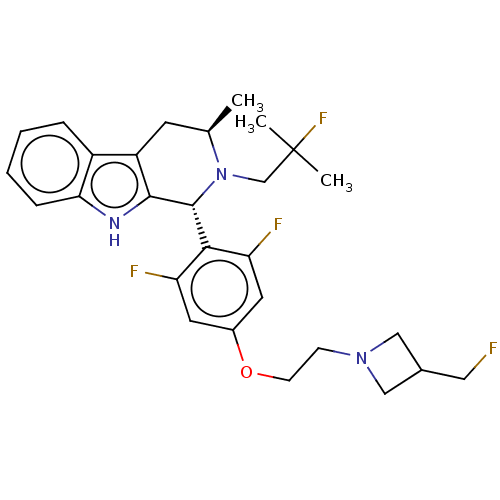 (CHEMBL4528514 | US11672785, Goodacre Compound 102 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572836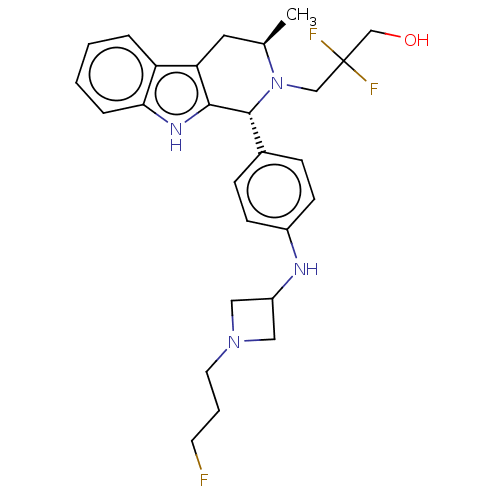 (CHEMBL4877406) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572812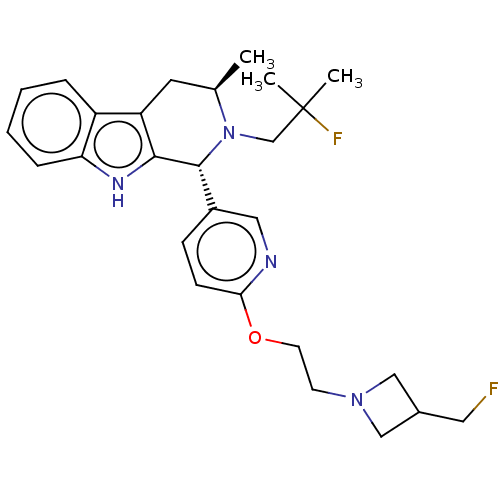 (CHEMBL4852984) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572816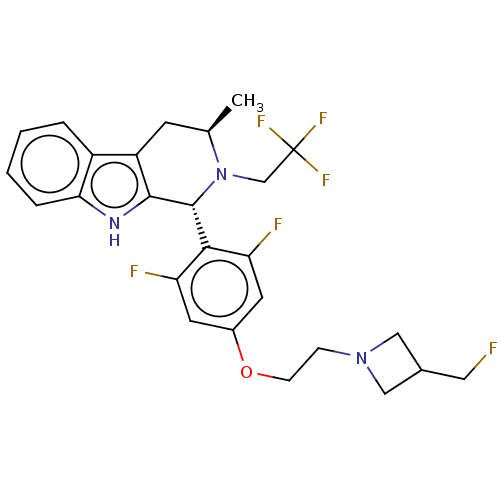 (CHEMBL4858543) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572818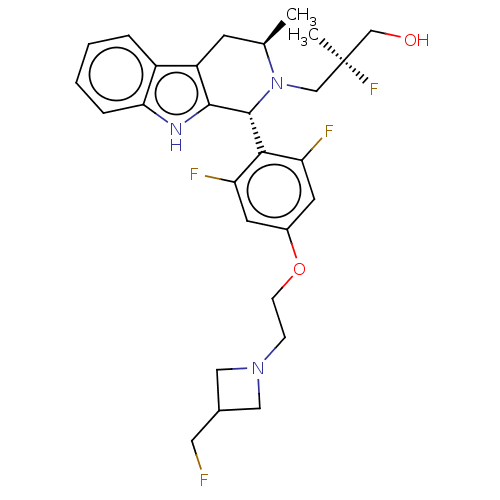 (CHEMBL4847664) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572830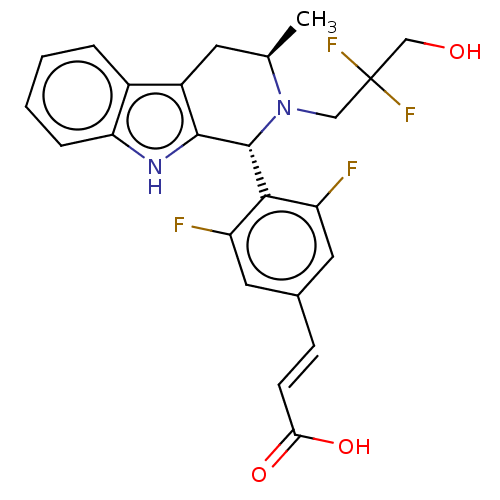 (CHEMBL4864337) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572835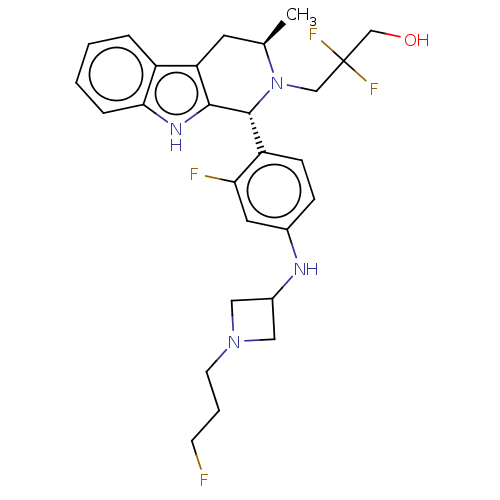 (CHEMBL4865515) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20608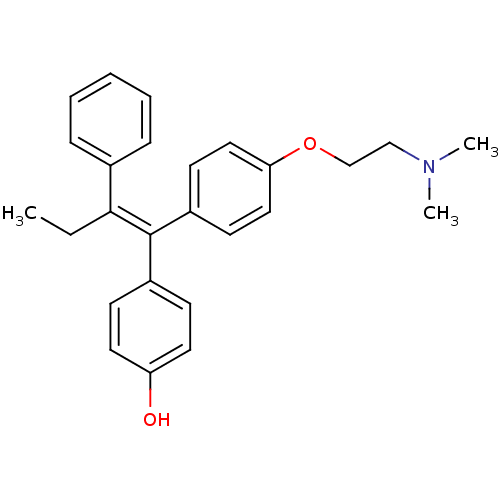 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572819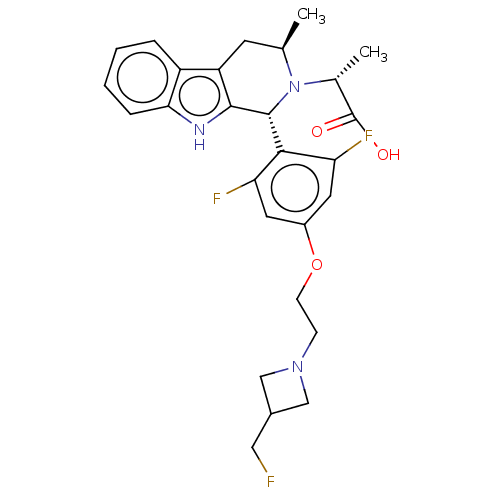 (CHEMBL4864628) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572810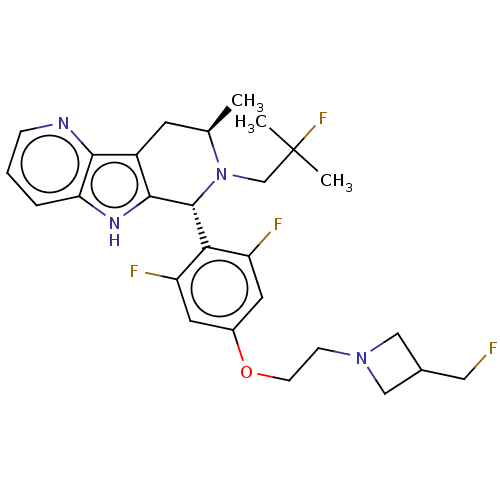 (CHEMBL4854999) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50090462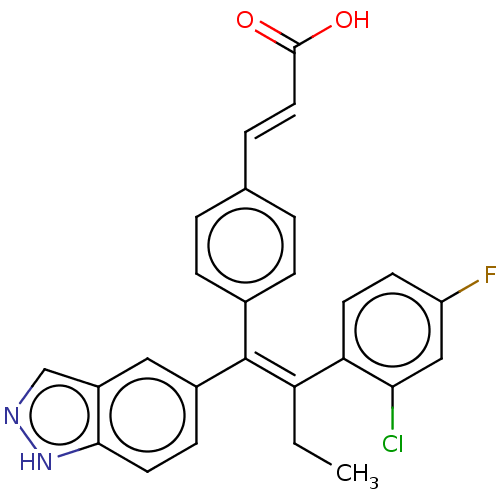 (CHEMBL3581693 | US20240043442, Example GDC-0810) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572813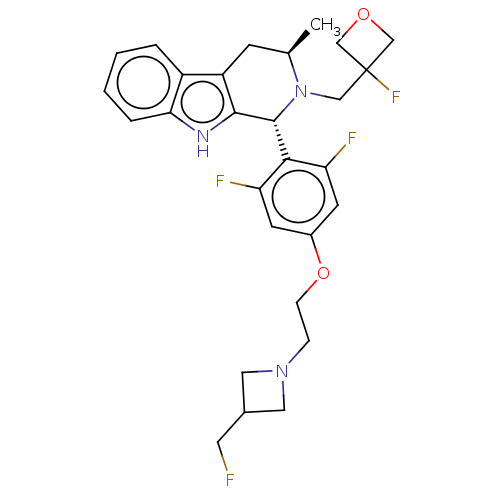 (CHEMBL4862431) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572811 (CHEMBL4869612) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20607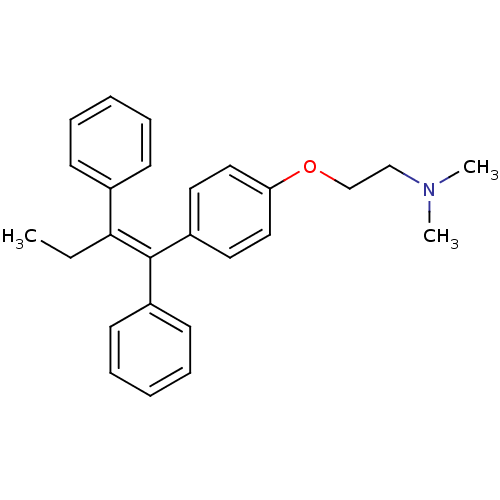 ((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50572837 (CHEMBL4877353) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at estrogen receptor in human T47D cells incubated for 18 hrs by ultra high sensitivity luminescence reporter gene assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50572809 (CHEMBL4866043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 10 mins followed by NADPH addition by LC/M... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50572809 (CHEMBL4866043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of CYP3A4 in human liver microsomes at 10 uM using midazolam as substrate preincubated for 10 mins followed by NADPH addition b... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50572809 (CHEMBL4866043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG expressed in HEK293 cells incubated for 5 mins by patch clamp assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50572808 (Gdc-9545 | Giredestrant | RO-7197597 | RO7197597) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG expressed in HEK293 cells incubated for 5 mins by patch clamp assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50572808 (Gdc-9545 | Giredestrant | RO-7197597 | RO7197597) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of CYP3A4 in human liver microsomes at 10 uM using midazolam as substrate preincubated for 10 mins followed by NADPH addition b... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50572809 (CHEMBL4866043) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 10 mins followed by NADPH addition by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50572808 (Gdc-9545 | Giredestrant | RO-7197597 | RO7197597) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of CYP2C9 in human liver microsomes using (s)-warfarin as substrate preincubated for 30 mins followed by NADPH addition by LC/M... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50572809 (CHEMBL4866043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of CYP2C9 in human liver microsomes using (s)-warfarin as substrate preincubated for 30 mins followed by NADPH addition by LC/M... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50572808 (Gdc-9545 | Giredestrant | RO-7197597 | RO7197597) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of CYP2C19 in human liver microsomes using (S)-Mephenytoin as substrate preincubated for 40 mins followed by NADPH addition by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50572809 (CHEMBL4866043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of CYP2C19 in human liver microsomes using (S)-Mephenytoin as substrate preincubated for 40 mins followed by NADPH addition by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50572808 (Gdc-9545 | Giredestrant | RO-7197597 | RO7197597) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 10 mins followed by NADPH addition by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50572808 (Gdc-9545 | Giredestrant | RO-7197597 | RO7197597) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Reversible inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 10 mins followed by NADPH addition by LC/M... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00847 BindingDB Entry DOI: 10.7270/Q20R9T67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||