

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579331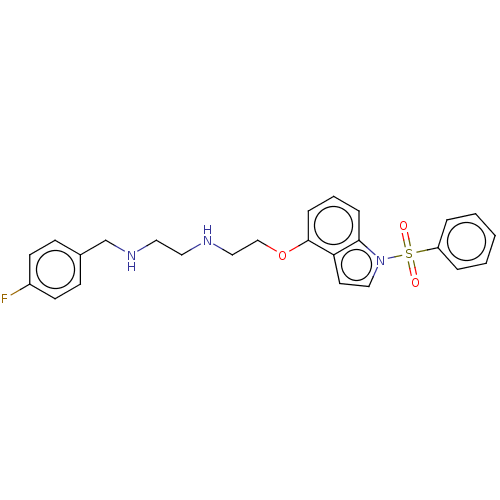 (CHEMBL4852099) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM78940 (METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579329 (CHEMBL4863218) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579335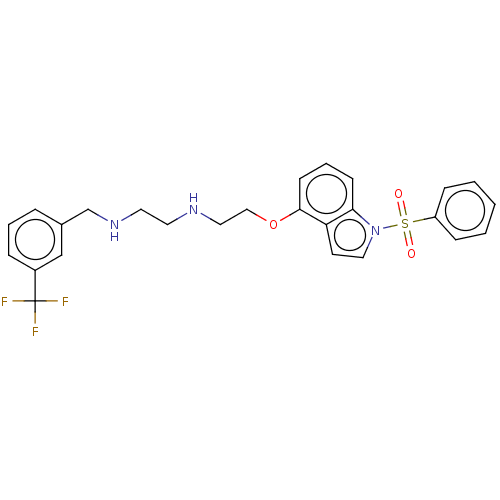 (CHEMBL4854972) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579327 (CHEMBL4847532) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579334 (CHEMBL4864206) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579332 (CHEMBL4851595) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579328 (CHEMBL4878383) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579323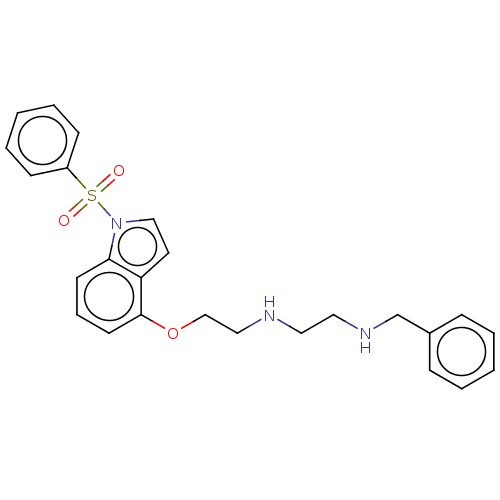 (CHEMBL4878361) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579320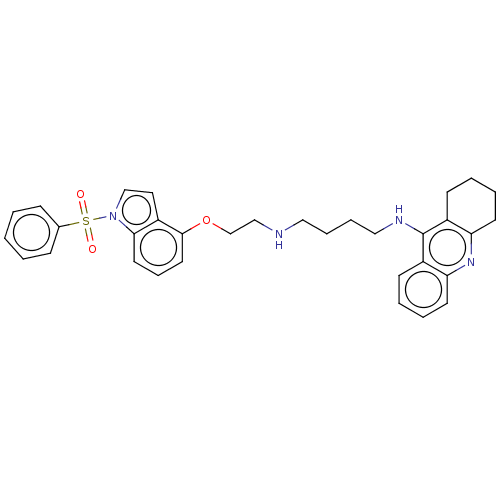 (CHEMBL4874513) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579333 (CHEMBL4868597) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579321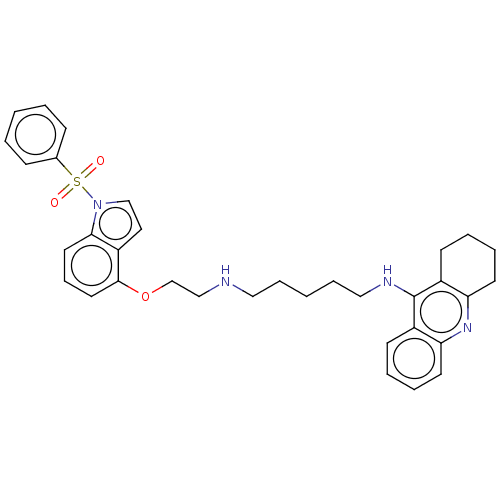 (CHEMBL4848231) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579324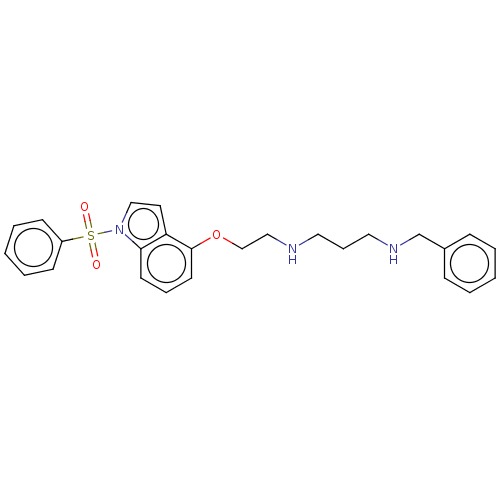 (CHEMBL4866788) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579325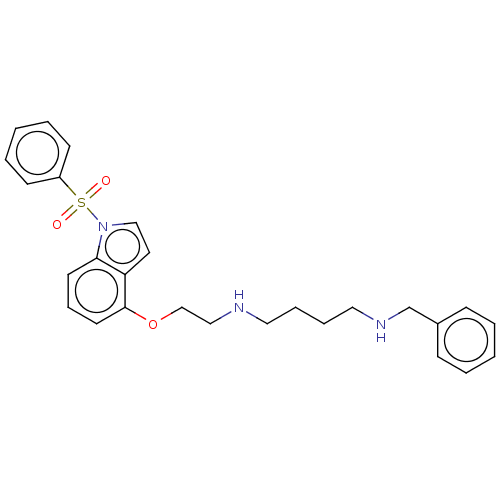 (CHEMBL4850488) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579322 (CHEMBL4877482) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579319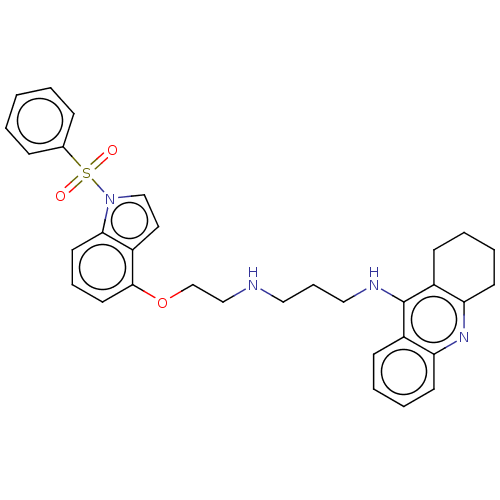 (CHEMBL4850051) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579330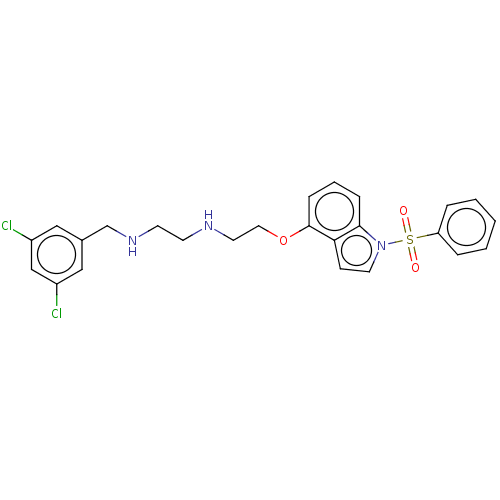 (CHEMBL4847373) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579326 (CHEMBL4871964) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579318 (CHEMBL4850546) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50579336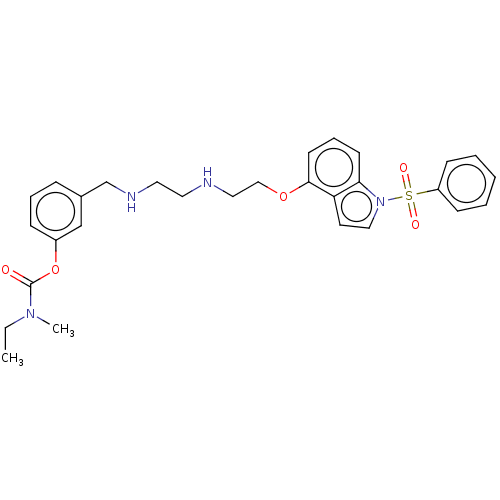 (CHEMBL4845823) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6R expressed in CHO-K1 cell membranes incubated for 1 hr by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579321 (CHEMBL4848231) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579322 (CHEMBL4877482) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50579318 (CHEMBL4850546) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition and measured after 5 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579320 (CHEMBL4874513) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition and measured after 5 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579319 (CHEMBL4850051) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50579319 (CHEMBL4850051) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition and measured after 5 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition and measured after 5 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579318 (CHEMBL4850546) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50579320 (CHEMBL4874513) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition and measured after 5 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50579322 (CHEMBL4877482) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition and measured after 5 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50579321 (CHEMBL4848231) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 5 mins followed by substrate addition and measured after 5 ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579326 (CHEMBL4871964) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 394 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579336 (CHEMBL4845823) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579323 (CHEMBL4878361) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 528 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579328 (CHEMBL4878383) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 586 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579324 (CHEMBL4866788) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 701 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579325 (CHEMBL4850488) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 887 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579334 (CHEMBL4864206) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579327 (CHEMBL4847532) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50579318 (CHEMBL4850546) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HFIP-pretreated human recombinant amyloid beta (1 to 42) self aggregation incubated up to 48 hrs under shaking condition and measured e... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579331 (CHEMBL4852099) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amyloid-beta precursor protein (Homo sapiens (Human)) | BDBM50579336 (CHEMBL4845823) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HFIP-pretreated human recombinant amyloid beta (1 to 42) self aggregation incubated up to 48 hrs under shaking condition and measured e... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579330 (CHEMBL4847373) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579329 (CHEMBL4863218) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579333 (CHEMBL4868597) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50579335 (CHEMBL4854972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine as substrate incubated for 5 mins followed by substrate addition and measured after 5 mins ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113783 BindingDB Entry DOI: 10.7270/Q21V5JSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 56 total ) | Next | Last >> |