

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315305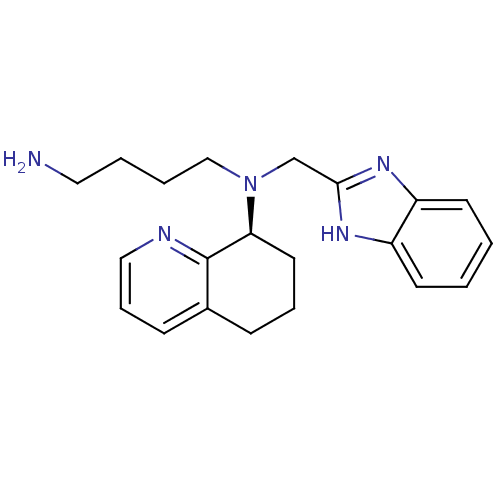 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541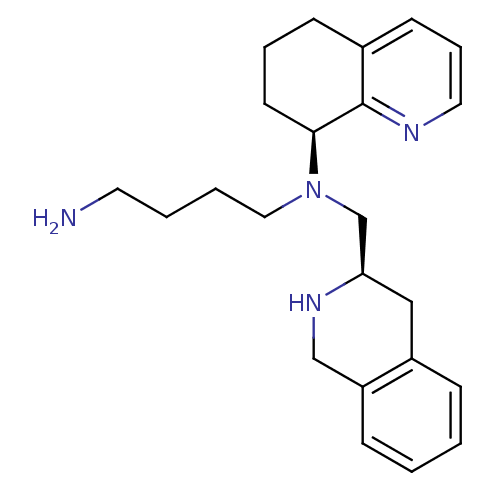 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579580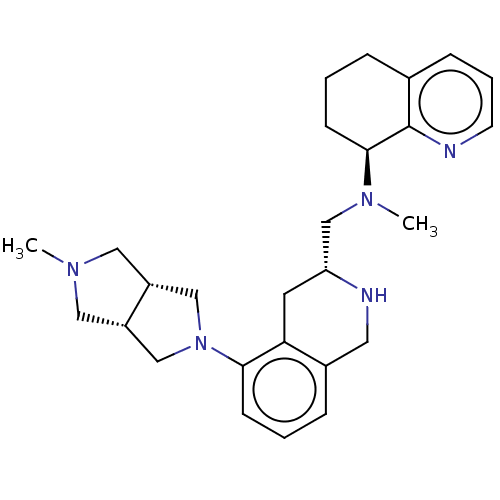 (CHEMBL4862509) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579579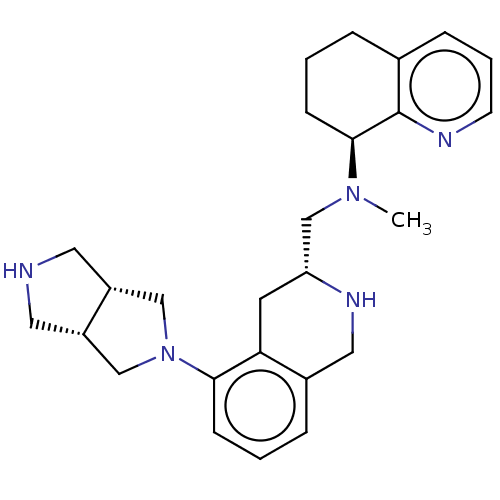 (CHEMBL4853570) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579583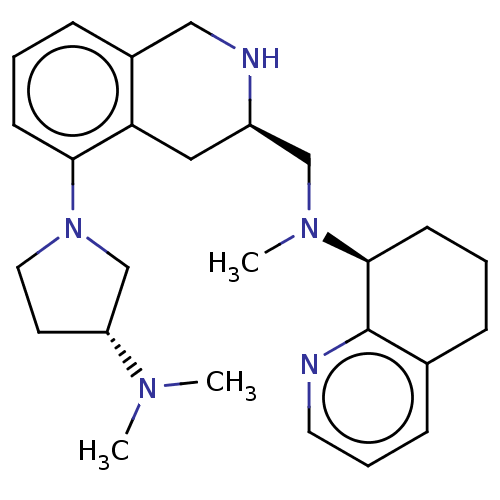 (CHEMBL4877757) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579582 (CHEMBL4875030) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579584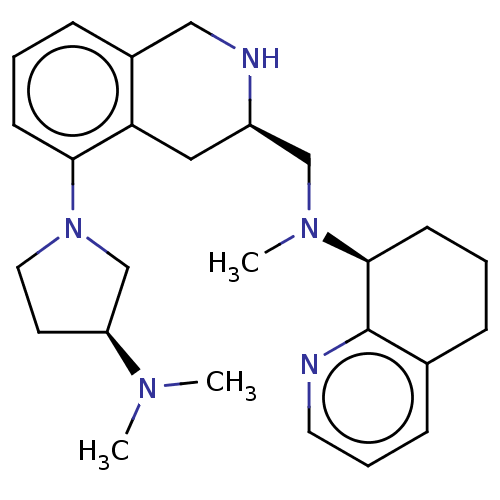 (CHEMBL4863276) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579576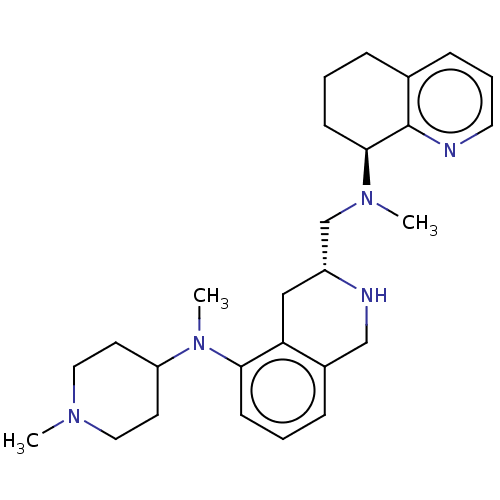 (CHEMBL4857452) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579585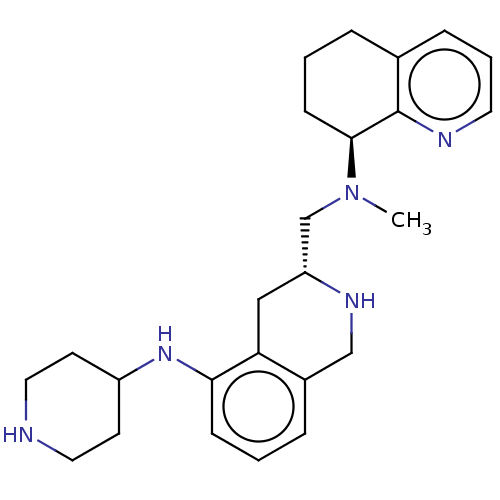 (CHEMBL4874526) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579581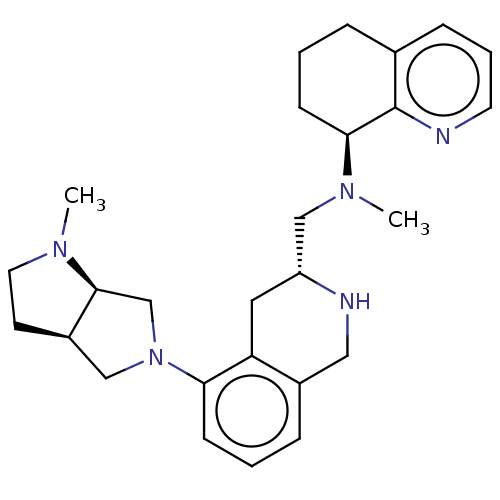 (CHEMBL4862362) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579574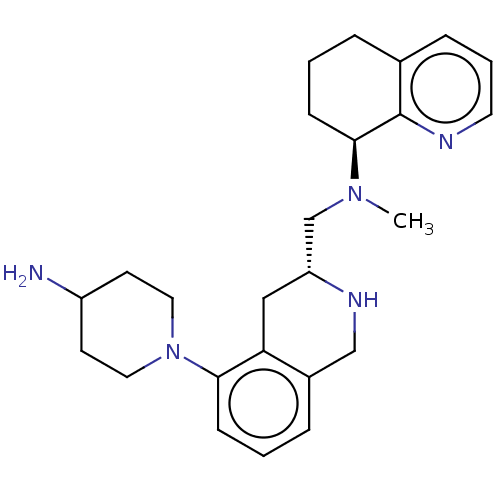 (CHEMBL4846946) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579577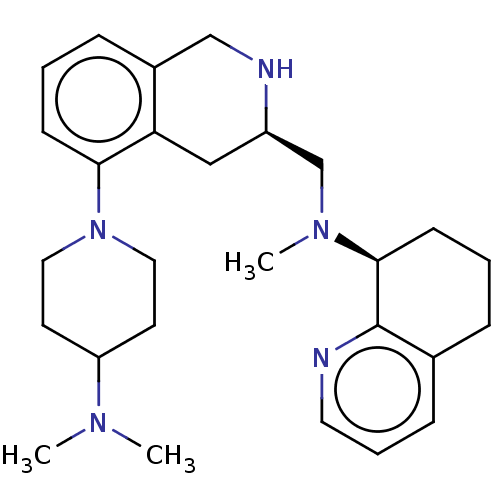 (CHEMBL4876951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579578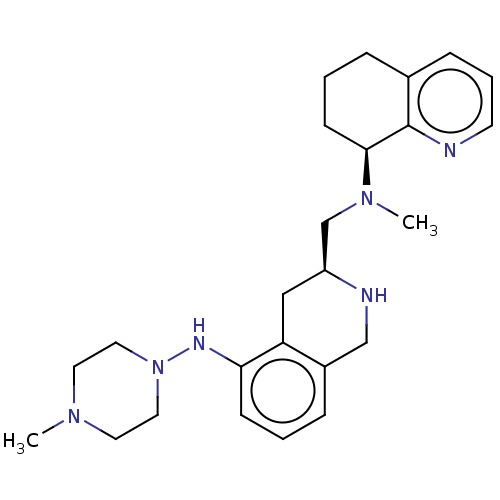 (CHEMBL4858136) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579575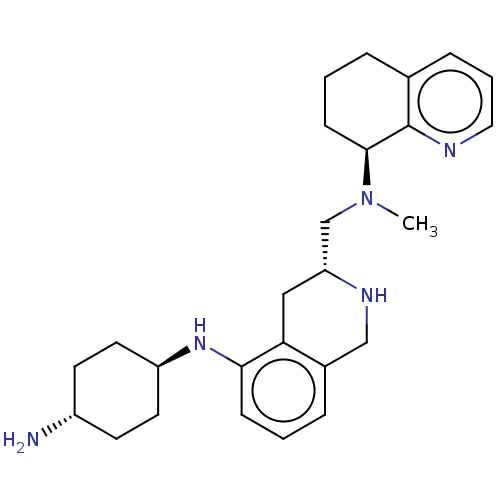 (CHEMBL4864332) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 755 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50315305 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50579573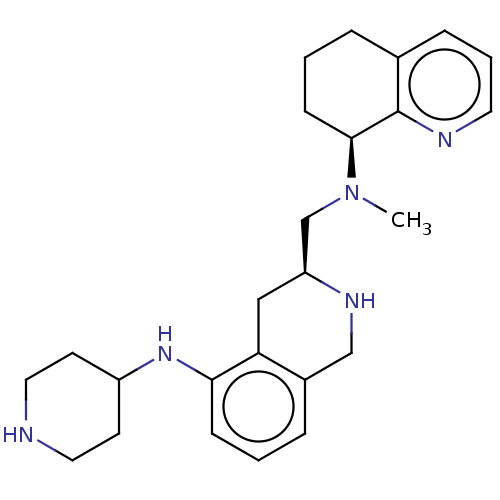 (CHEMBL4849088) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CXCR4 receptor in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579583 (CHEMBL4877757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579574 (CHEMBL4846946) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50579577 (CHEMBL4876951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP2C9 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50579578 (CHEMBL4858136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human ERG by automated patch clamp method | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579579 (CHEMBL4853570) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579584 (CHEMBL4863276) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579581 (CHEMBL4862362) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579582 (CHEMBL4875030) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579585 (CHEMBL4874526) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579573 (CHEMBL4849088) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579575 (CHEMBL4864332) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579576 (CHEMBL4857452) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579577 (CHEMBL4876951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579578 (CHEMBL4858136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579580 (CHEMBL4862509) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579585 (CHEMBL4874526) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50315305 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579574 (CHEMBL4846946) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579579 (CHEMBL4853570) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579581 (CHEMBL4862362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579582 (CHEMBL4875030) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579583 (CHEMBL4877757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50579584 (CHEMBL4863276) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of CYP3A4 (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579573 (CHEMBL4849088) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579580 (CHEMBL4862509) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579578 (CHEMBL4858136) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579575 (CHEMBL4864332) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579576 (CHEMBL4857452) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50579577 (CHEMBL4876951) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CYP2D6 expressed in insect cells using AMMC as substrate preincubated for 30 mins followed by NADPH regenerating syst... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00449 BindingDB Entry DOI: 10.7270/Q23N277W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||