

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196149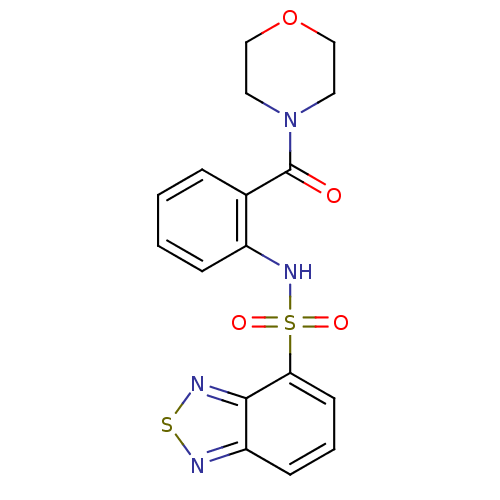 (4-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196150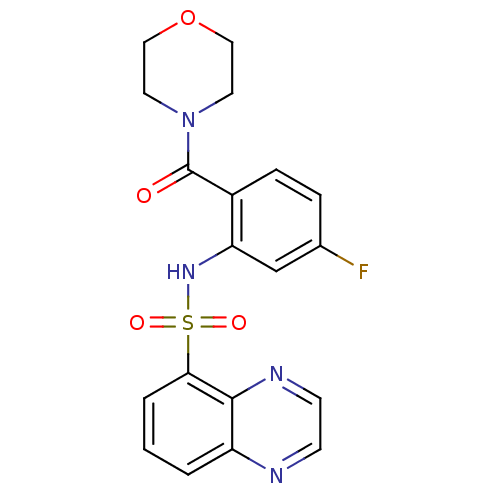 (4-[4-fluoro-2-[(5-quinoxalinylsulfonyl)amino]benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196151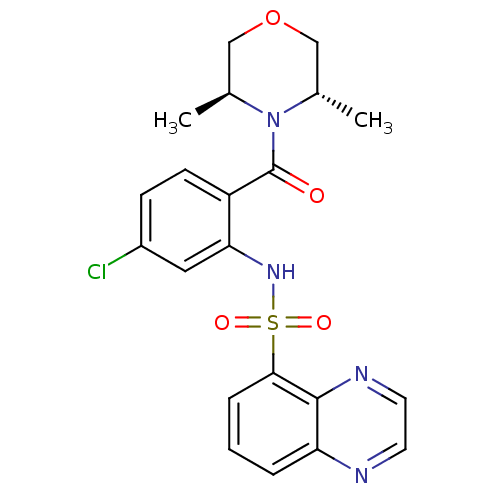 ((3S,5S)-4-[4-chloro-2-[(5-quinoxalinylsulfonyl)ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196152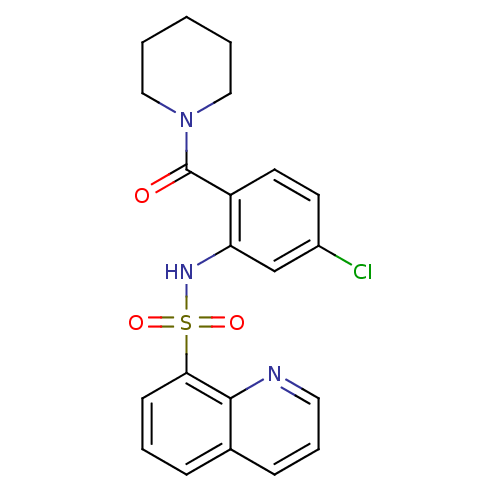 (1-[4-chloro-2-[(quinolin-8-ylsulfonyl)amino]-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50196155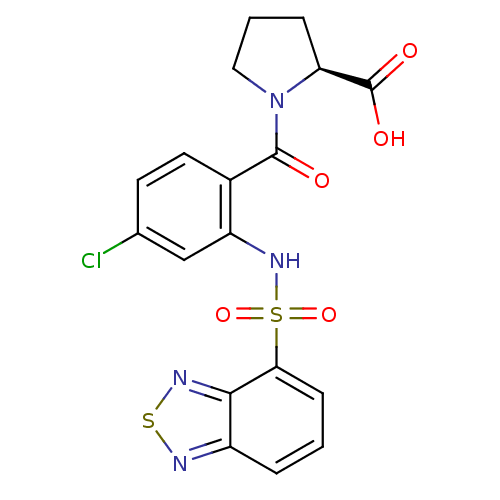 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK2R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196154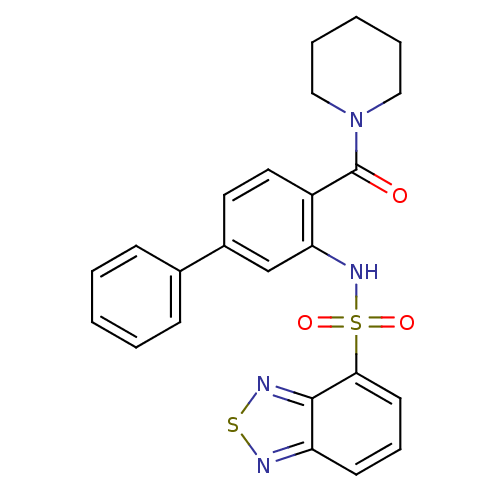 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50196153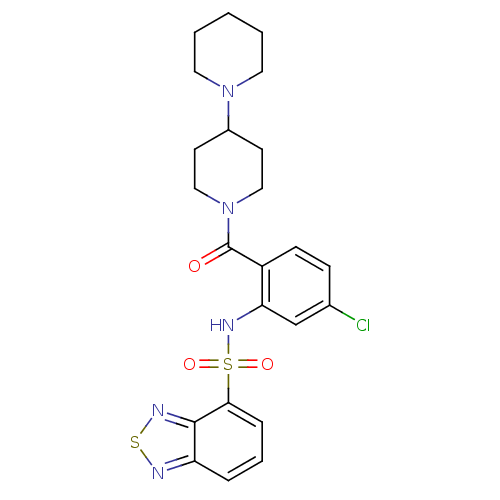 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK2R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50185262 ((+/-)-2-(2-(1H-indole-2-carboxamido)benzamido)-3-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK2R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196156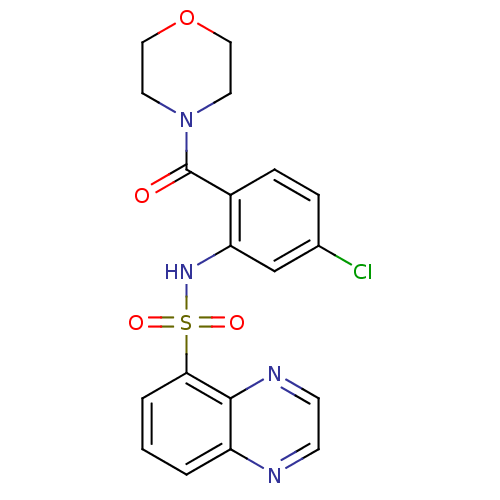 (4-[4-chloro-2-[(5-quinoxalinylsulfonyl)amino]benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196157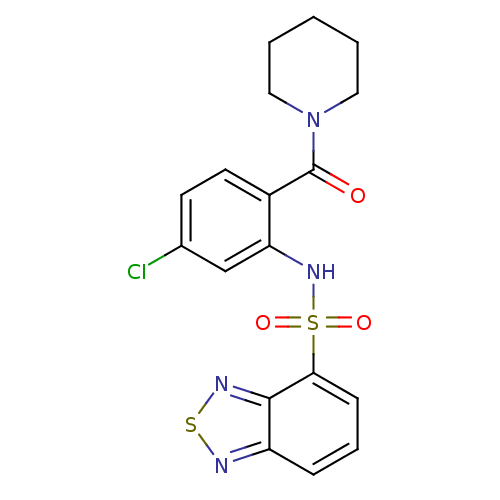 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196159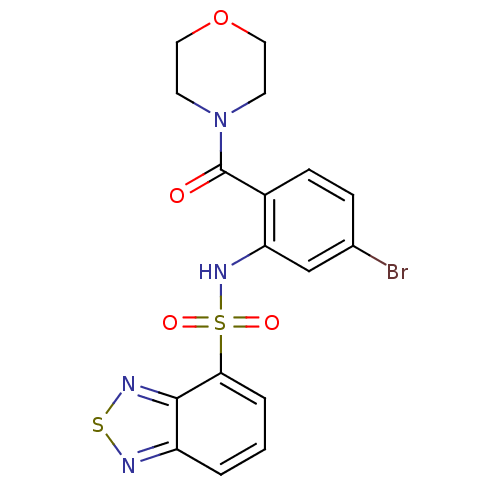 (4-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196158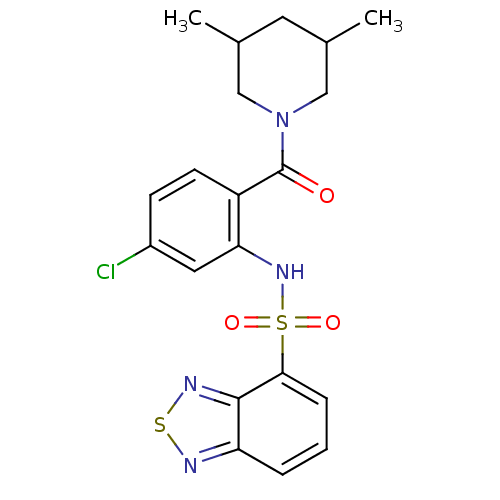 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50196160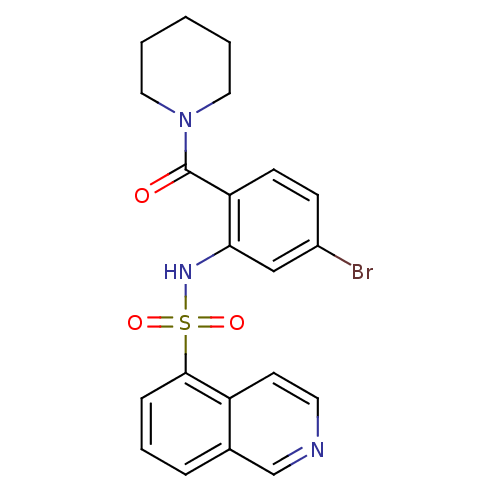 (1-[4-bromo-2-[(isoquinolin-5-ylsulfonyl)amino]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK2R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196161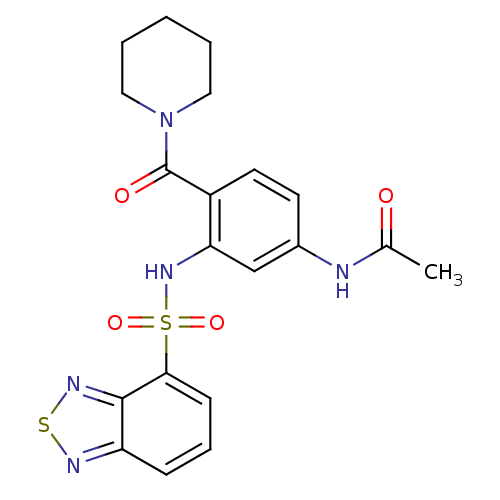 (1-[4-acetylamino-2-[(2,1,3-benzothiadiazol-4-ylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196162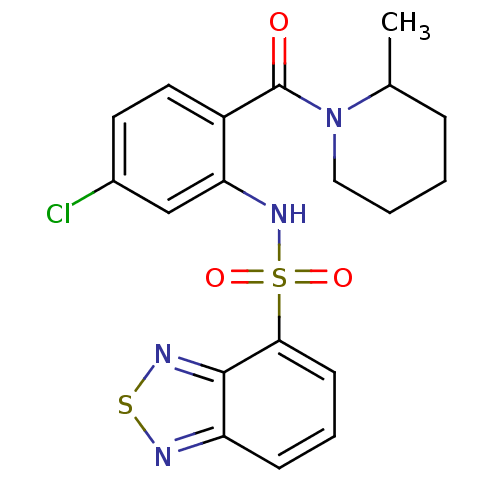 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196163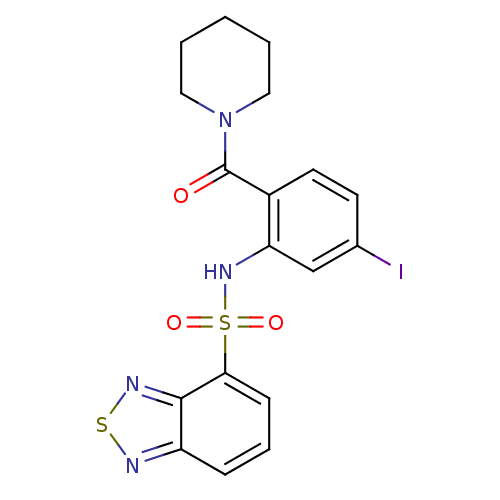 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196160 (1-[4-bromo-2-[(isoquinolin-5-ylsulfonyl)amino]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196155 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50196164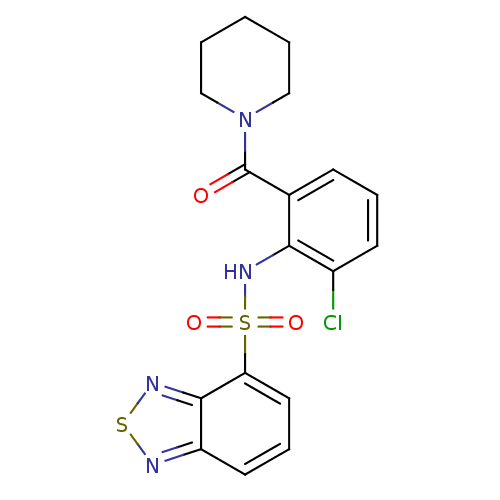 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK2R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50196166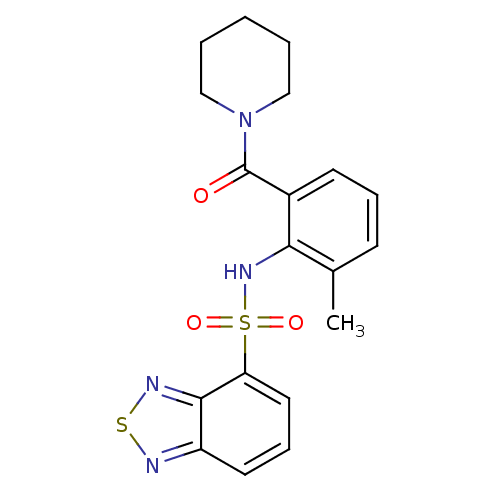 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK2R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196165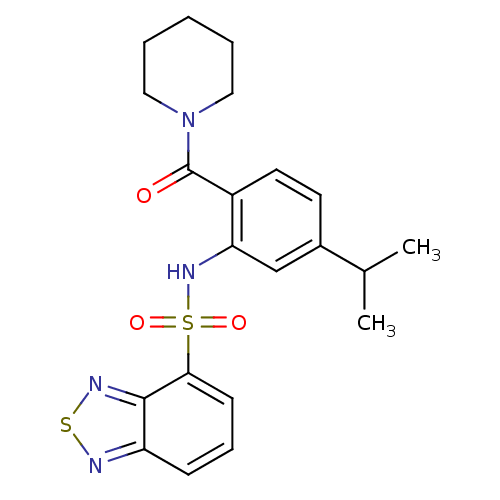 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50196173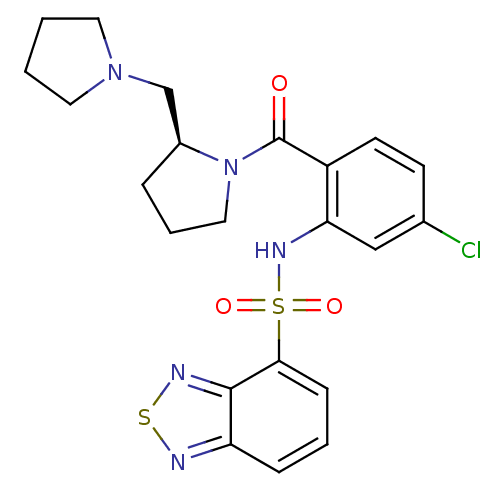 ((S)-1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK2R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196167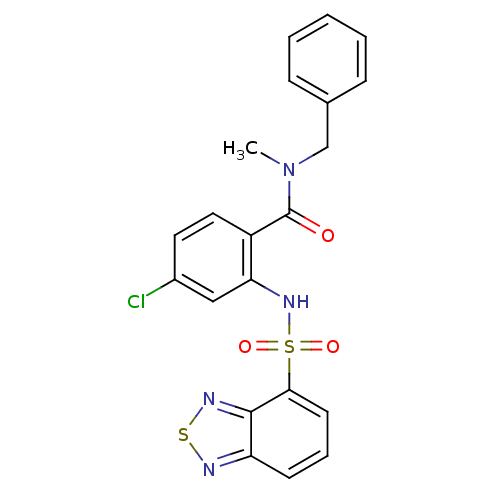 (2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4-ch...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196168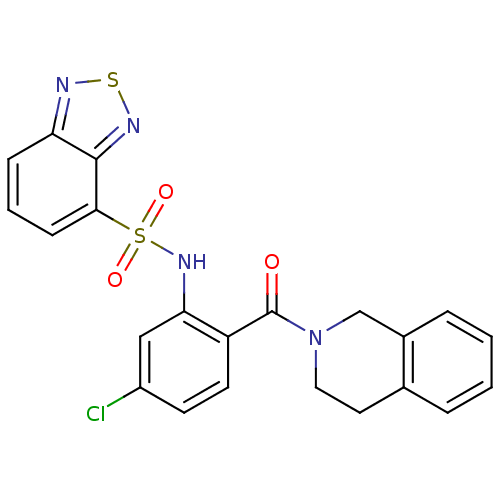 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196174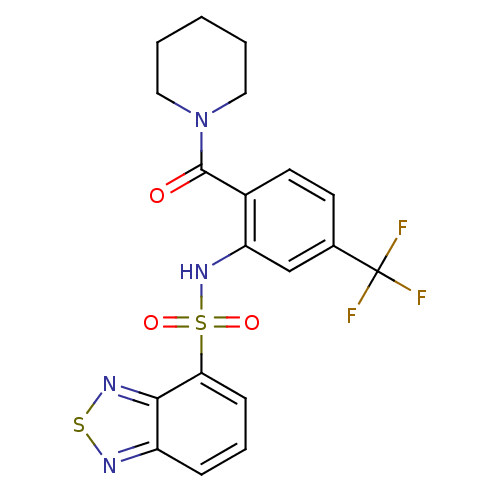 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196172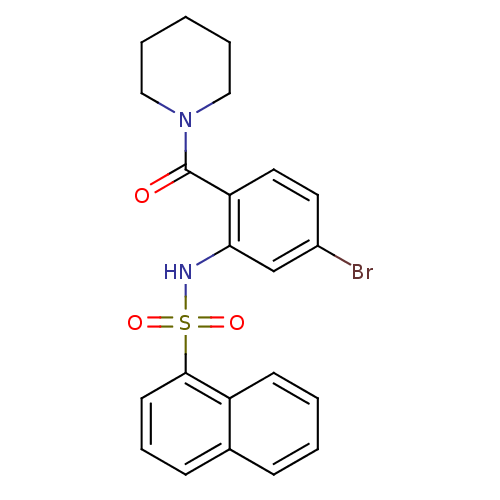 (1-[4-bromo-2-[[naphthalen-1-ylsulfonyl]amino]benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196169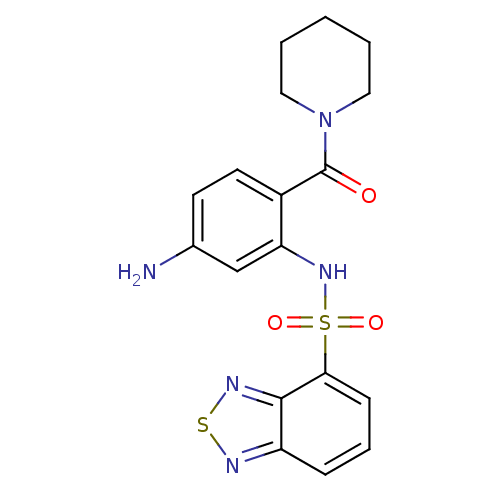 (1-[4-amino-2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196170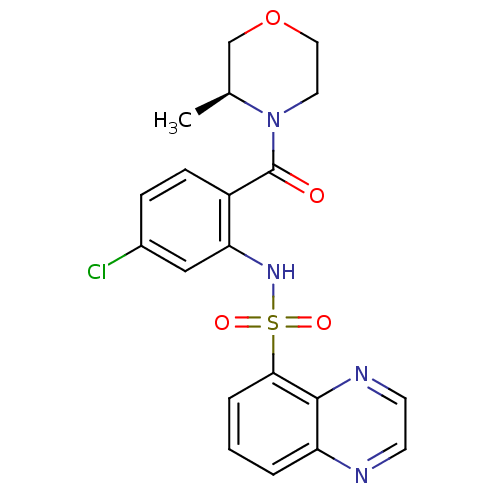 ((S)-4-[4-chloro-2-[(5-quinoxalinylsulfonyl)amino]b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196171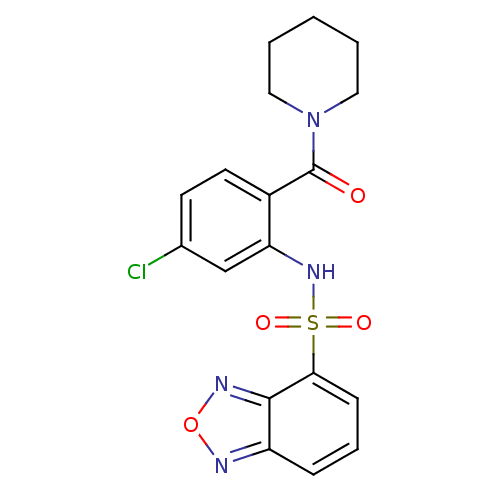 (1-[2-[(2,1,3-benzooxadiazol-4-ylsulfonyl)amino]-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196176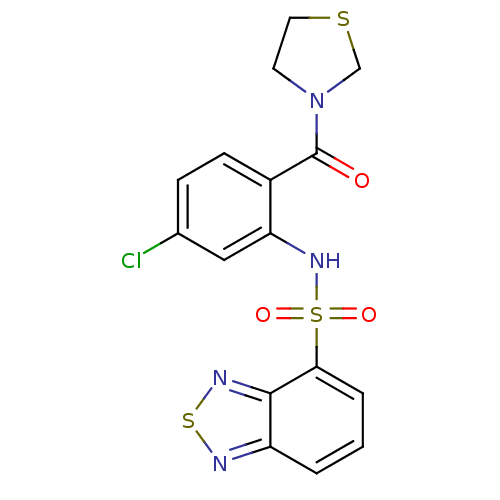 (3-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196175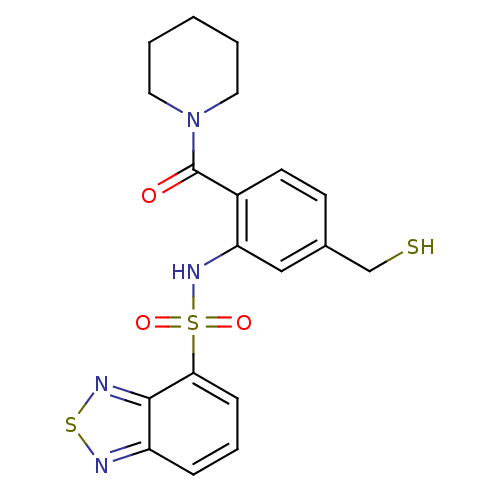 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196177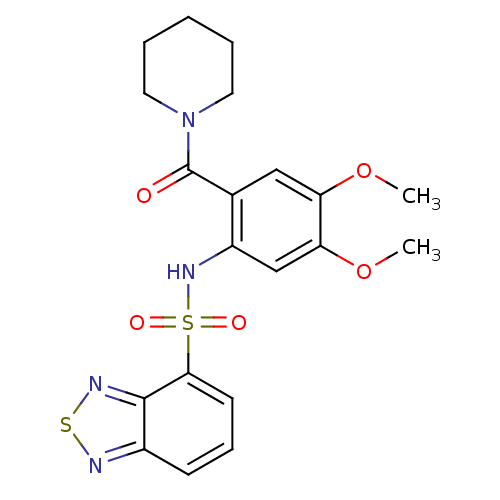 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196180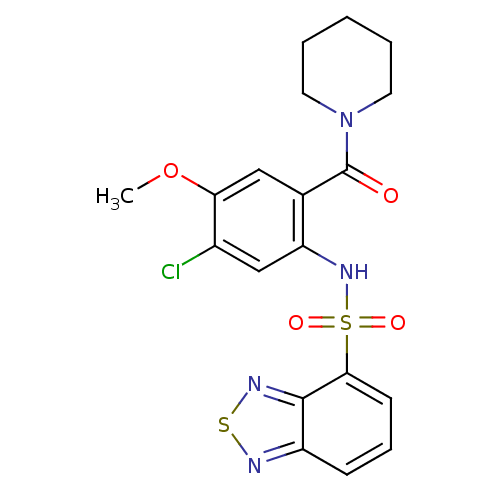 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196178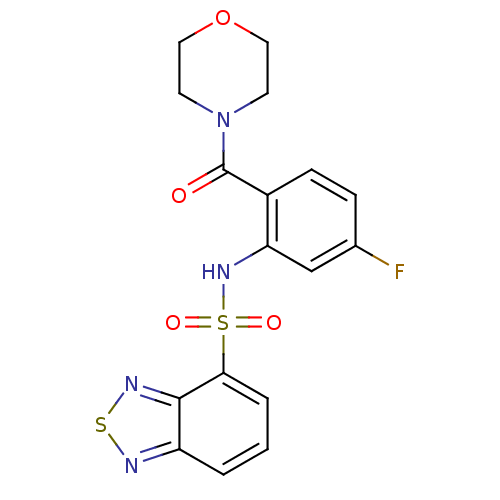 (4-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196179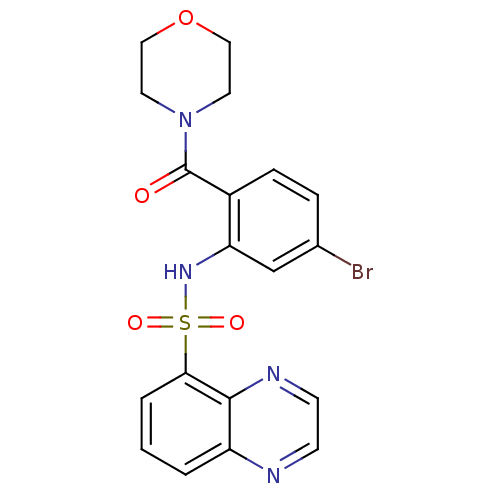 (4-[4-bromo-2-[(5-quinoxalinylsulfonyl)amino]benzoy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196186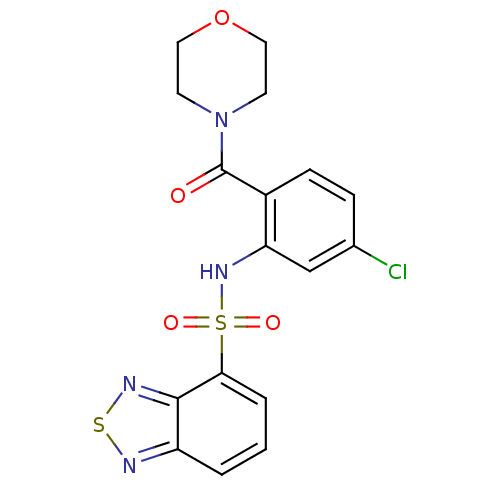 (4-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196185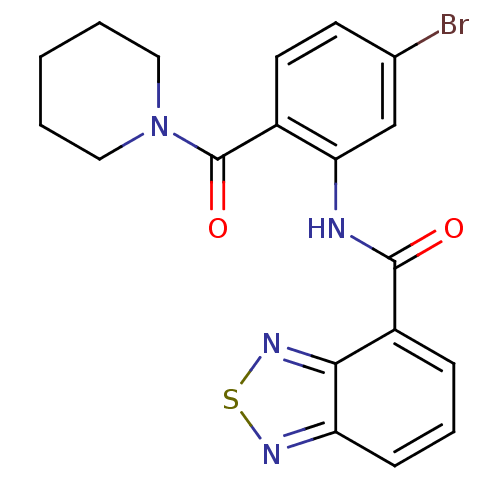 (1-[2-[(2,1,3-benzothiadiazol-4-ylcarbonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196188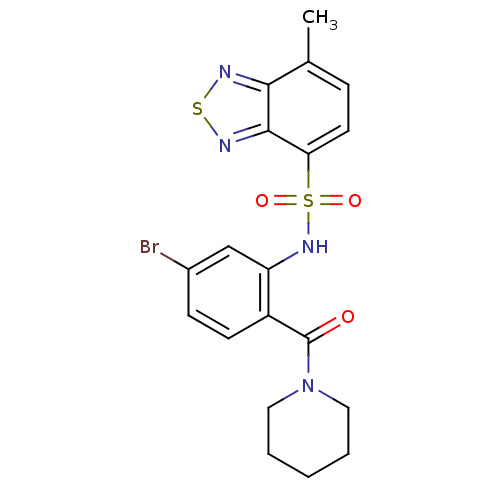 (1-[4-bromo-2-[7-methyl(2,1,3-benzothiadiazol-4-yls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196187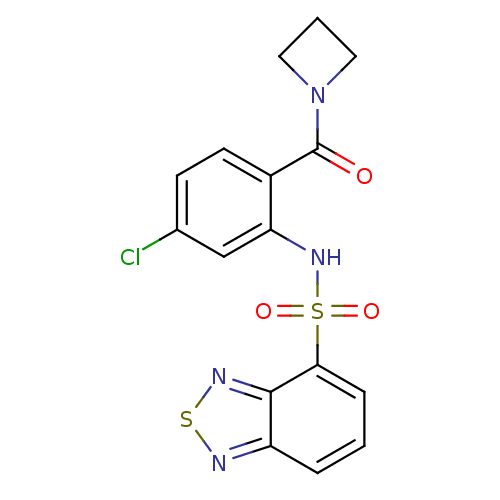 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196173 ((S)-1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196190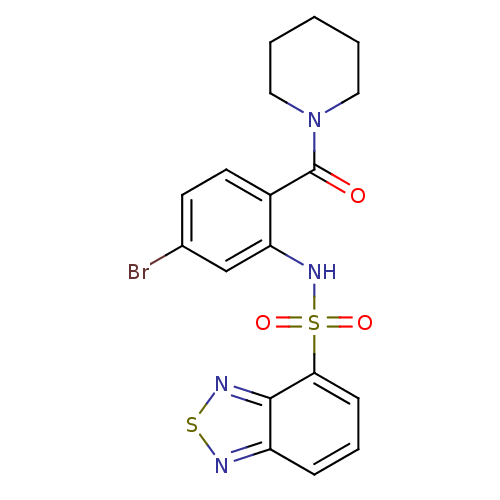 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196184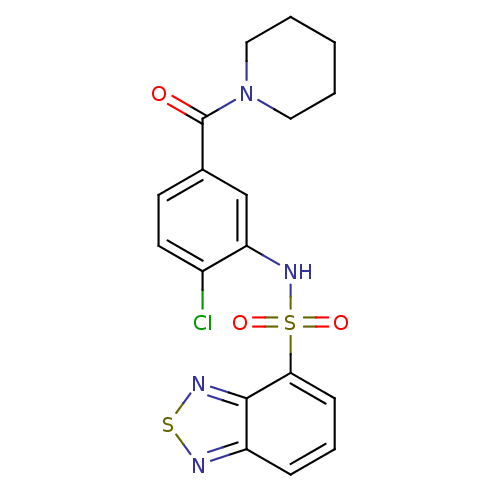 (1-[3-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196166 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196181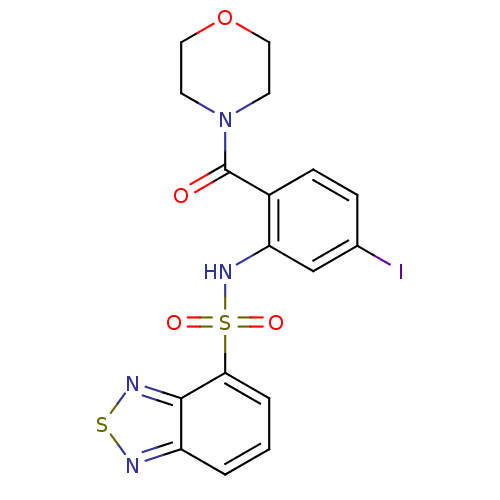 (4-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196182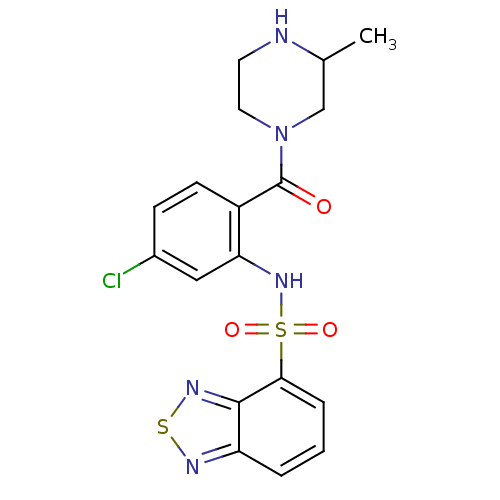 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196183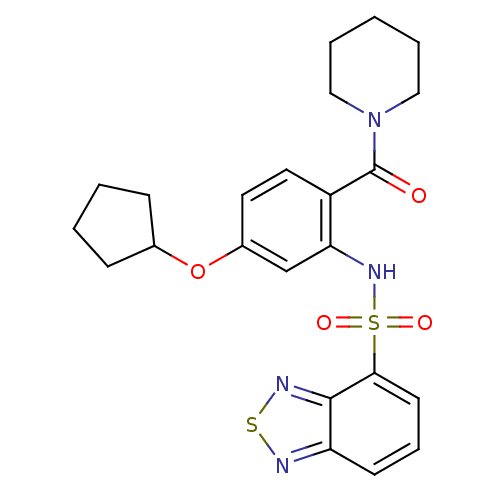 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196189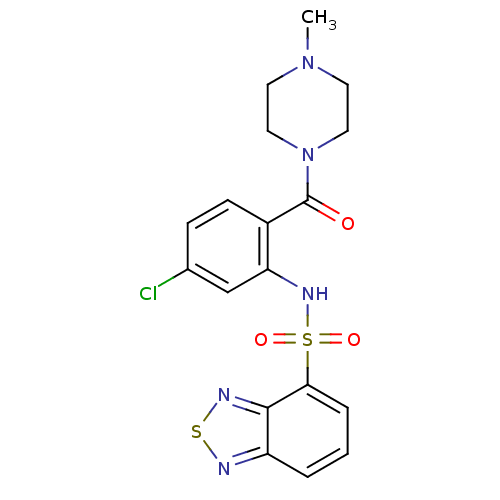 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50196182 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK2R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196193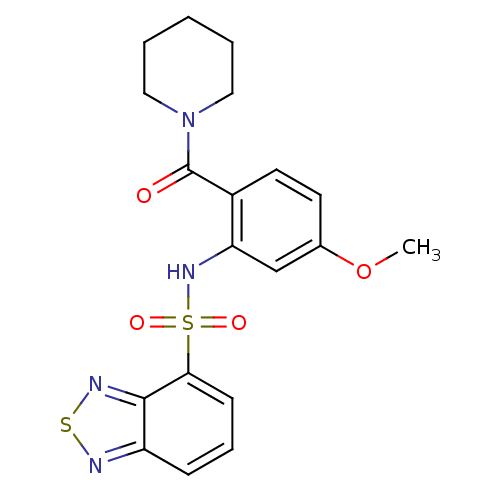 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)amino]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50196194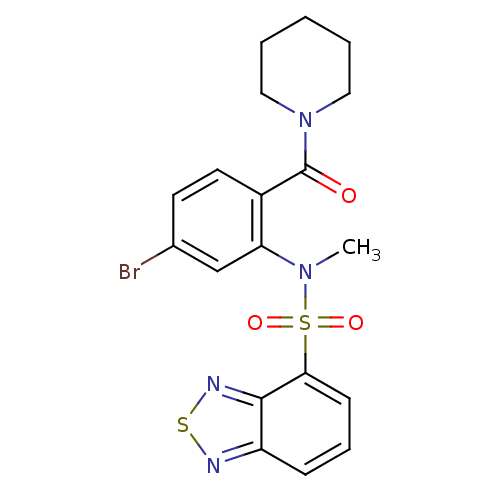 (1-[2-[(2,1,3-benzothiadiazol-4-ylsulfonyl)methylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]CCK-8S from human CCK1R | J Med Chem 49: 6371-90 (2006) Article DOI: 10.1021/jm060590x BindingDB Entry DOI: 10.7270/Q2MK6CHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |