

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297172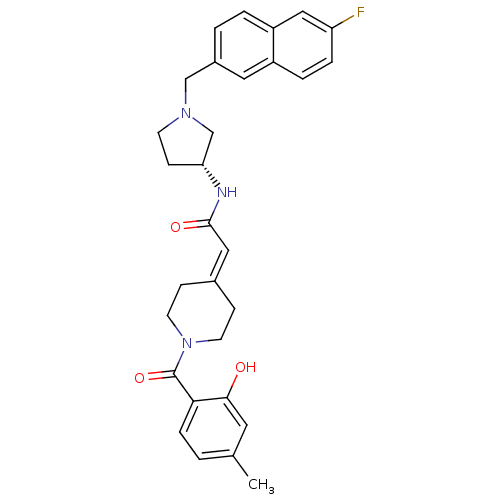 (CHEMBL560275 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297171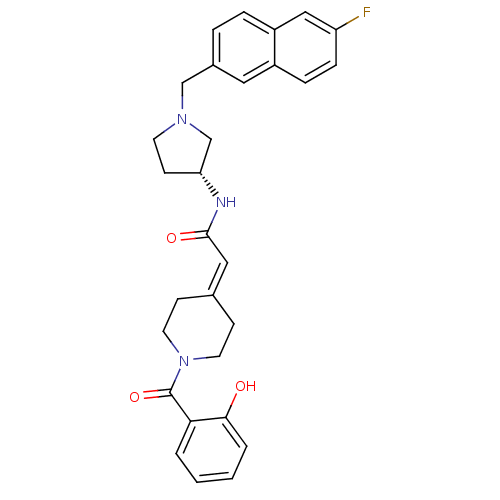 (CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells by functional inhibition curve analysis | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297181 (2-[1-(1,3-Benzodioxol-5-ylcarbonyl)piperidin-4-yli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297183 (CHEMBL551738 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297177 (CHEMBL561535 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297182 (2-[1-(3,4-Dimethoxybenzoyl)piperidin-4-ylidene]-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297190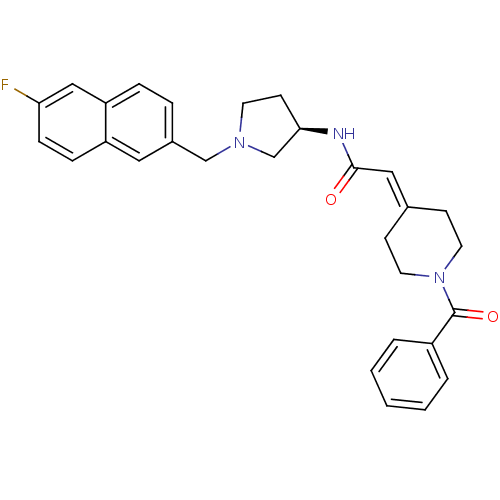 (2-(1-Benzoylpiperidin-4-ylidene)-N-{(3R)-1-[(6-flu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297188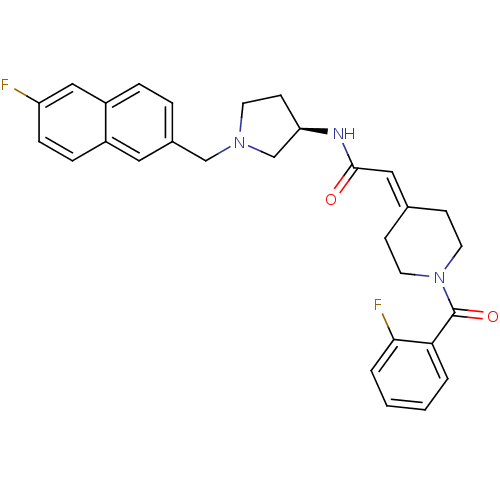 (2-[1-(2-Fluorobenzoyl)piperidin-4-ylidene]-N-{(3R)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297180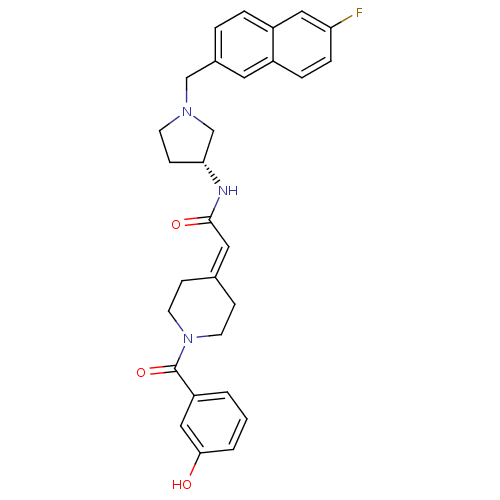 (CHEMBL556916 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297185 (CHEMBL556227 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297179 (CHEMBL562923 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297184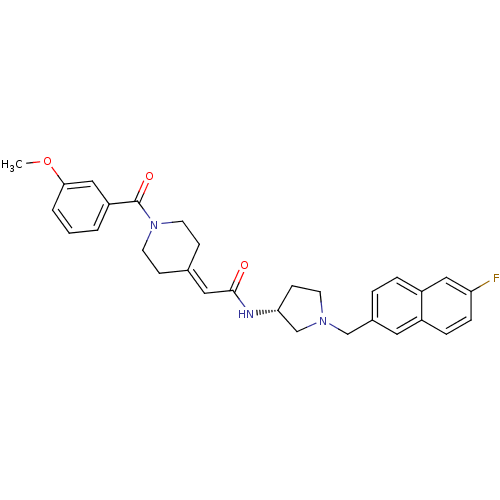 (CHEMBL557118 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297174 (2-[1-(4-Fluoro-2-hydroxybenzoyl)piperidin-4-yliden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297173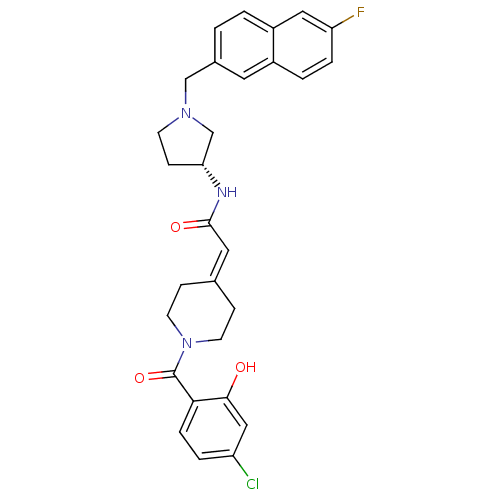 (2-[1-(4-Chloro-2-hydroxybenzoyl)piperidin-4-yliden...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297171 (CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297187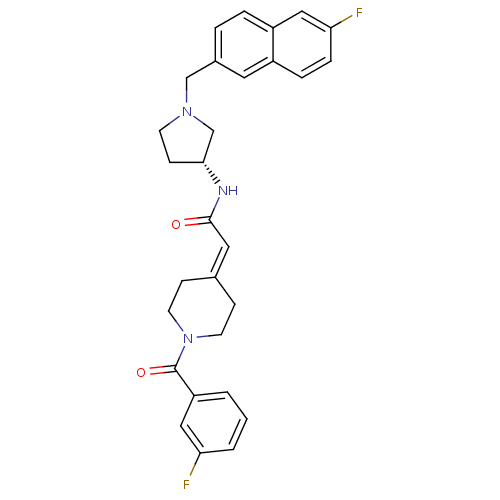 ((R)-2-(1-(3-fluorobenzoyl)piperidin-4-ylidene)-N-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297176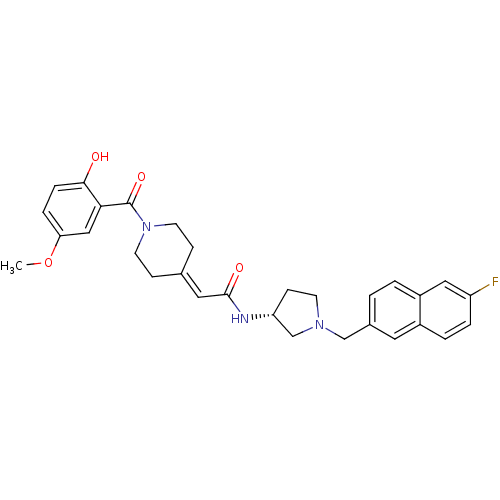 (CHEMBL562574 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50264142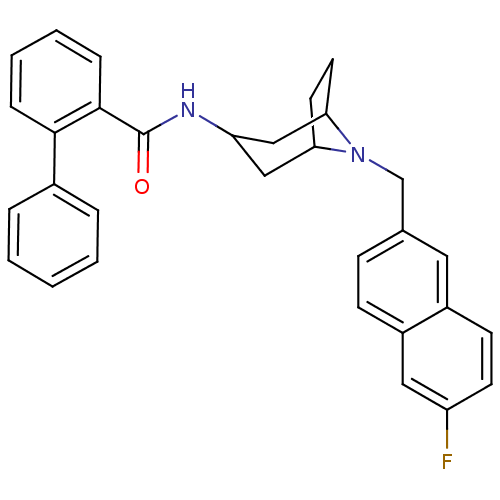 (Biphenyl-2-carboxylic acid [(1R,5R)-8-(6-fluoro-na...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264142 (Biphenyl-2-carboxylic acid [(1R,5R)-8-(6-fluoro-na...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297186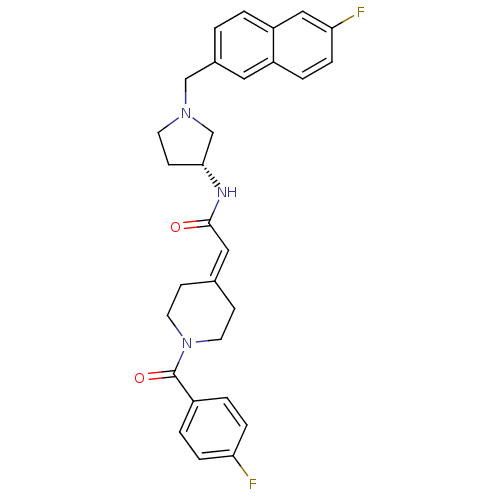 (2-[1-(4-Fluorobenzoyl)piperidin-4-ylidene]-N-{(3R)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50264212 (CHEMBL519042 | N-{(3-exo)-8-[(6-Fluoro-2-naphthyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50225349 (CHEMBL409830 | N-(1-((6-fluoronaphthalen-2-yl)meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297178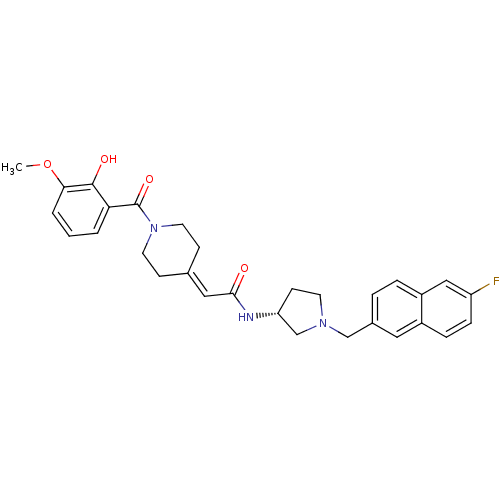 (CHEMBL565165 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297175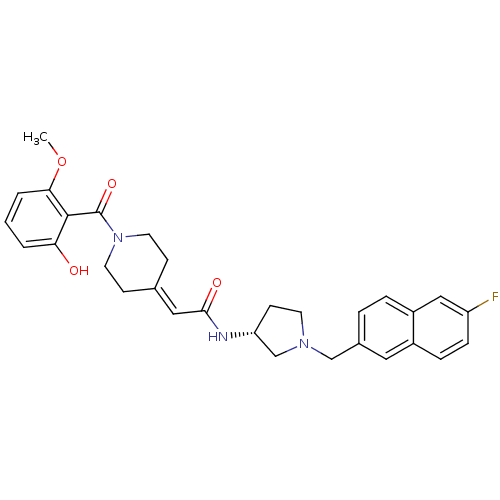 (CHEMBL562922 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297195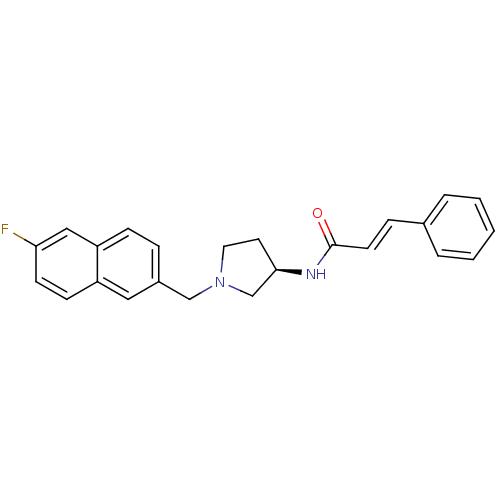 ((2E)-N-{(3R)-1-[(6-fluoro-2-naphthyl)methyl]pyrrol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297199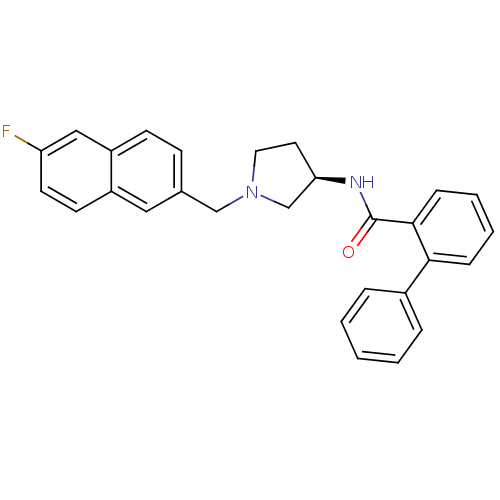 (CHEMBL562475 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297189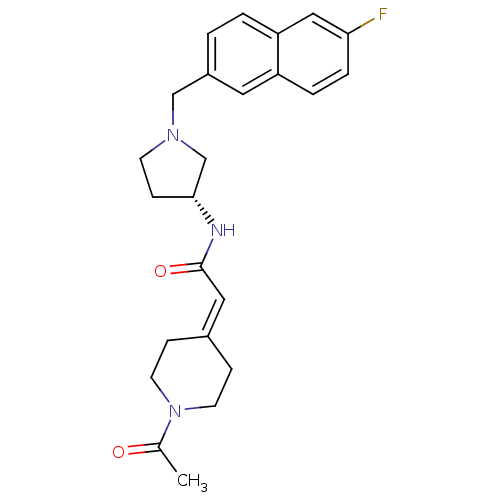 (2-(1-Acetylpiperidin-4-ylidene)-N-{(3R)-1-[(6-fluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297197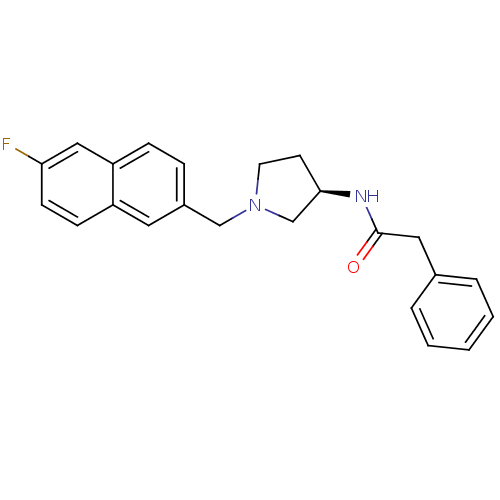 (CHEMBL549514 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297192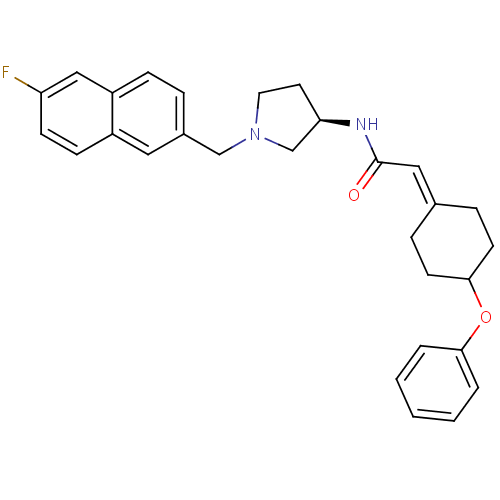 (CHEMBL560135 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable C-C chemokine receptor type 3 (Mus musculus) | BDBM50297171 (CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of mouse CCR3 | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297198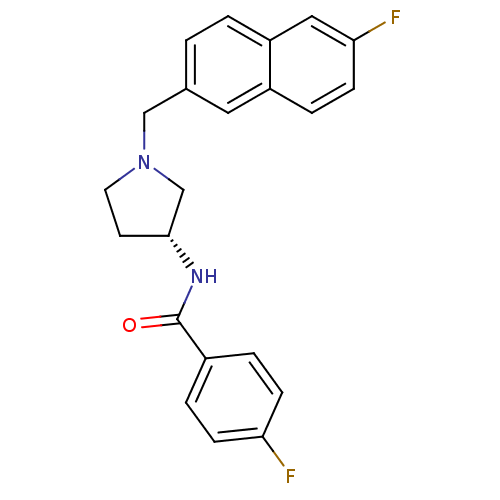 (4-Fluoro-N-{(3R)-1-[(6-fluoro-2-naphthyl)methyl]-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297194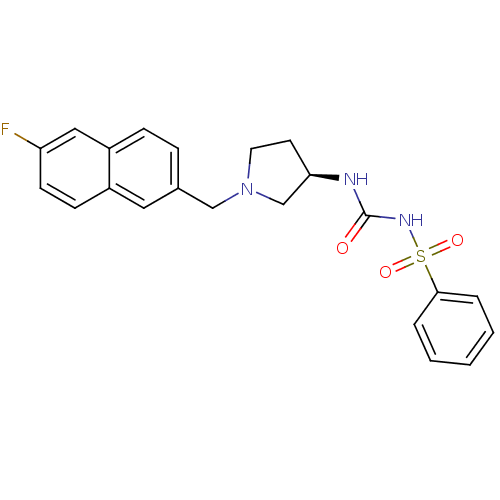 (CHEMBL563019 | N-({(3R)-1-[(6-Fluoro-2-naphthyl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297196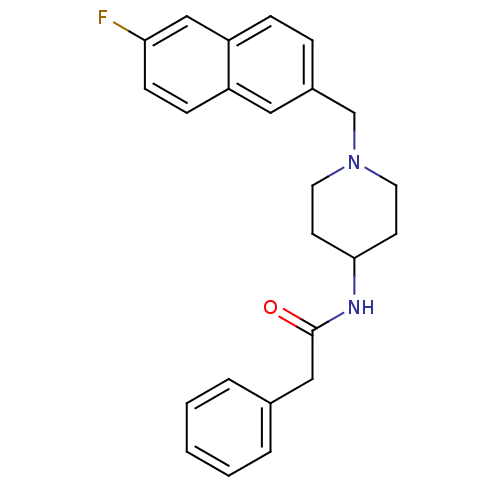 (CHEMBL557936 | N-{1-[(6-Fluoro-2-naphthyl)methyl]p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297201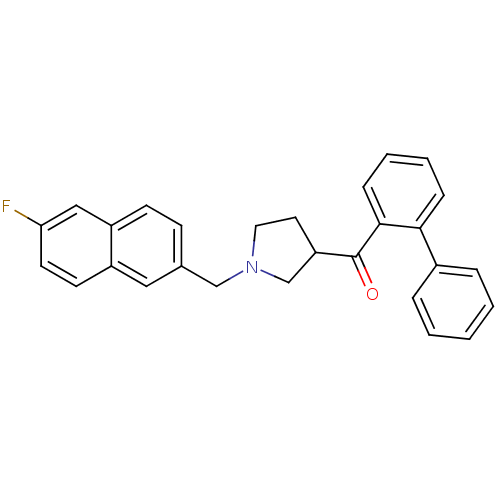 (CHEMBL561941 | N-{(rac)-1-[(6-Fluoro-2-naphthyl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297202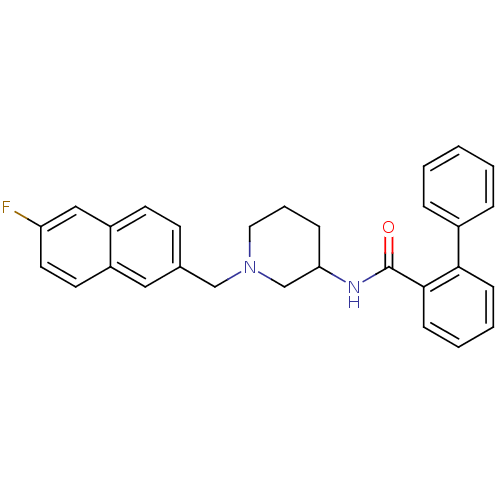 ((rac)-N-{1-[(6-Fluoro-2-naphthyl)methyl]piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50225353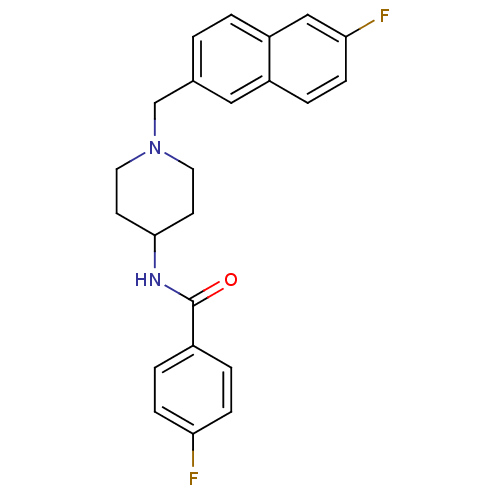 (4-fluoro-N-(1-((6-fluoronaphthalen-2-yl)methyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297191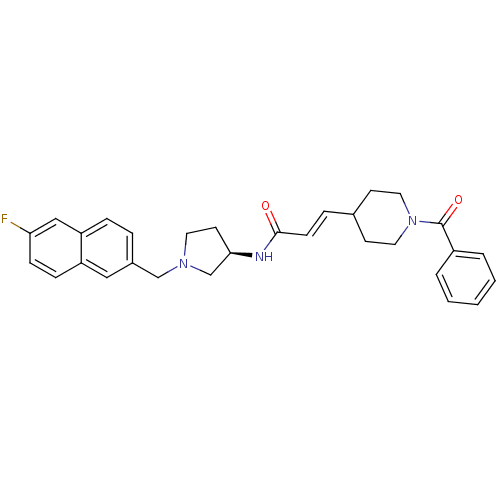 ((2E)-3-(1-Benzoylpiperidin-4-yl)-N-{(3R)-1-[(6-flu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable C-C chemokine receptor type 3 (Mus musculus) | BDBM50264212 (CHEMBL519042 | N-{(3-exo)-8-[(6-Fluoro-2-naphthyl)...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of mouse CCR3 | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297193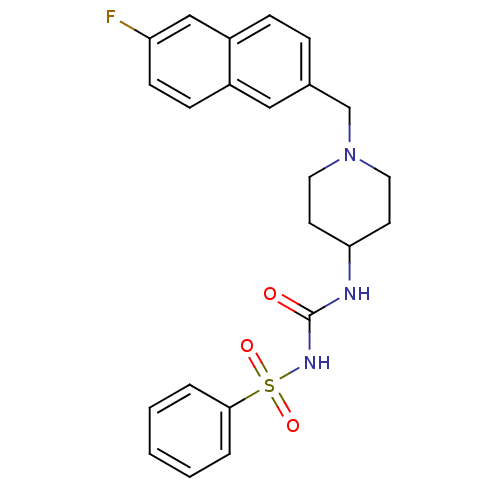 (CHEMBL551205 | N-({1-[(6-Fluoro-2-naphthyl)methyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50297200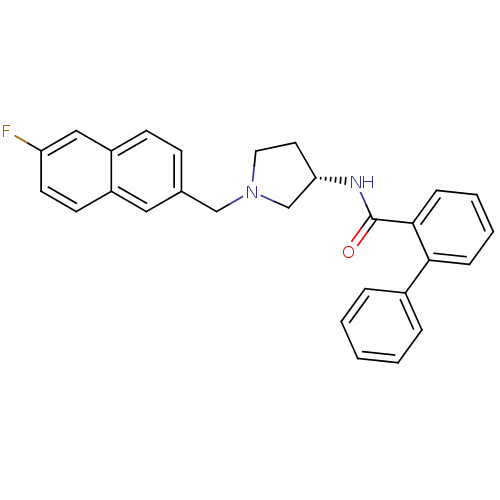 (CHEMBL563319 | N-{(3S)-1-[(6-Fluoro-2-naphthyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50225356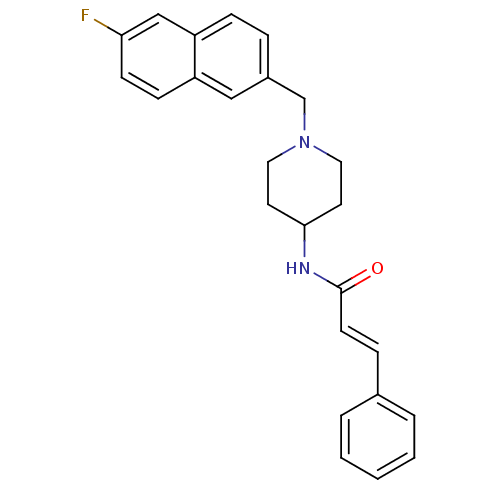 ((2E)-N-{1-[(6-fluoro-2-naphthyl)methyl]piperidin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR3 expressed in mouse B300-19 cells assessed as inhibition of eotaxin-induced calcium flux | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50297171 (CHEMBL551735 | N-{(3R)-1-[(6-Fluoro-2-naphthyl)met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50264212 (CHEMBL519042 | N-{(3-exo)-8-[(6-Fluoro-2-naphthyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem 17: 5989-6002 (2009) Article DOI: 10.1016/j.bmc.2009.06.066 BindingDB Entry DOI: 10.7270/Q2WD40NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||