

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50056999 (CHEMBL56367 | nimesulide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of purified COX2 assessed as formation of oxidized TMPD during reduction og PGG2 to PGH2 preincubated for 15 mins by chromogenic assay | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343920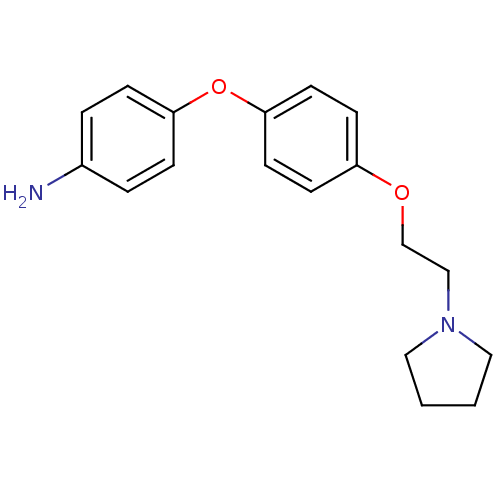 (4-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)aniline | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085245 (1-[2-(4-Phenoxy-phenoxy)-ethyl]-pyrrolidine | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343916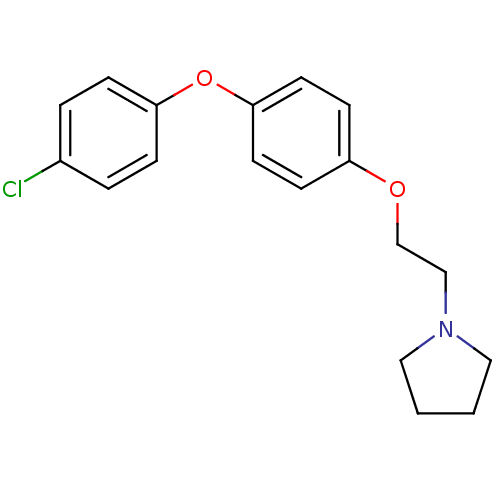 (1-(2-(4-(4-Chlorophenoxy)phenoxy)ethyl)pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343915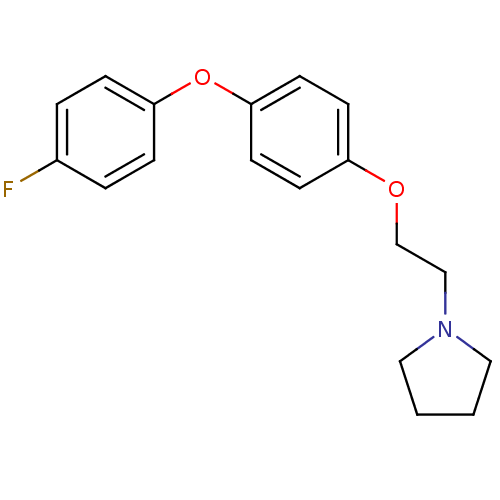 (1-(2-(4-(4-Fluorophenoxy)phenoxy)ethyl)pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343918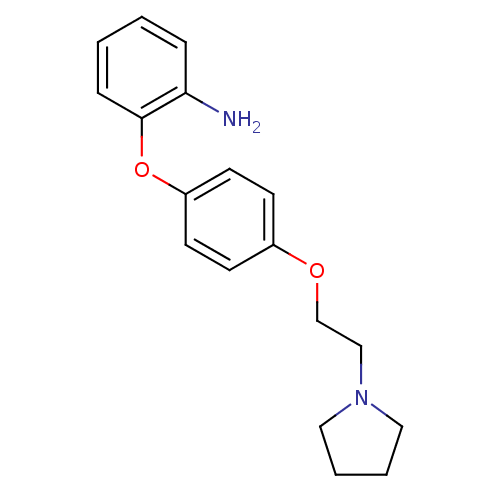 (2-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)aniline | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343917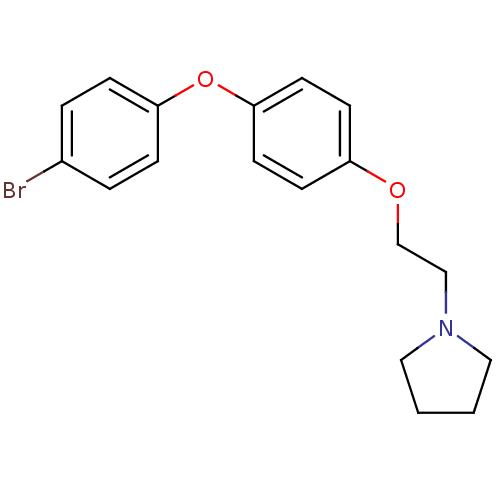 (1-(2-(4-(4-Bromophenoxy)phenoxy)ethyl)pyrrolidine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343904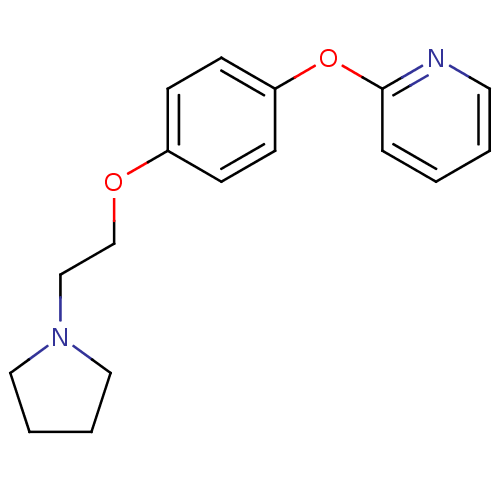 (2-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)pyridine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343912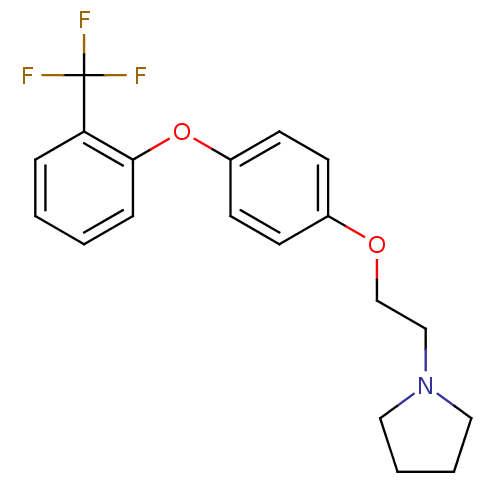 (1-(2-(4-(2-(Trifluoromethyl)phenoxy)phenoxy)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343917 (1-(2-(4-(4-Bromophenoxy)phenoxy)ethyl)pyrrolidine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343920 (4-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)aniline | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343927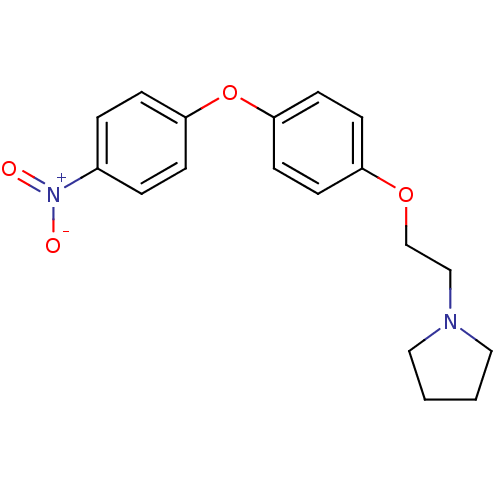 (1-(2-(4-(4-Nitrophenoxy)phenoxy)ethyl)pyrrolidine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343914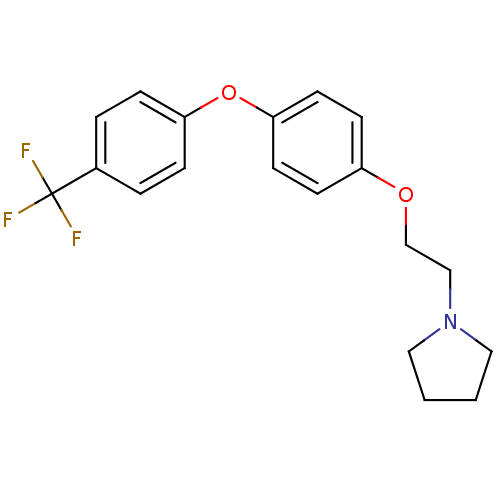 (1-(2-(4-(4-(Trifluoromethyl)phenoxy)phenoxy)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343926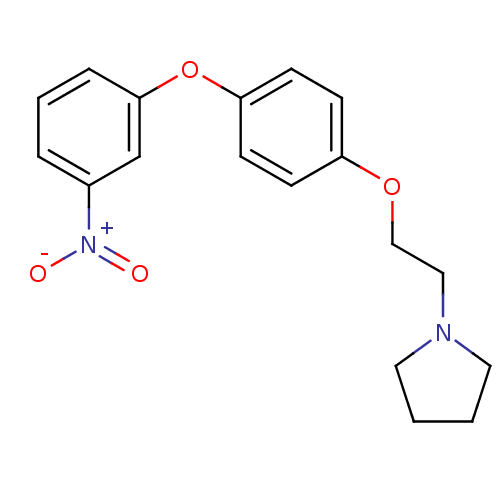 (1-(2-(4-(3-Nitrophenoxy)phenoxy)ethyl)pyrrolidine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343913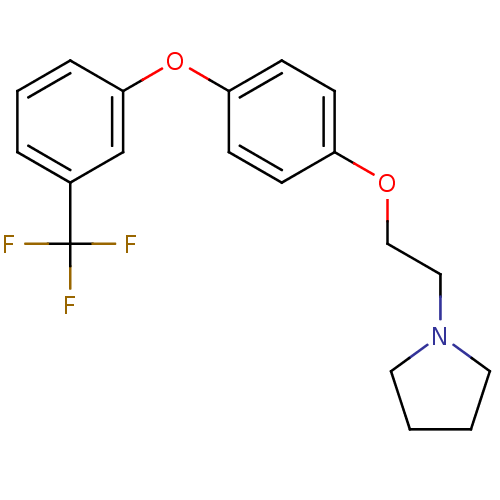 (1-(2-(4-(3-(Trifluoromethyl)phenoxy)phenoxy)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343905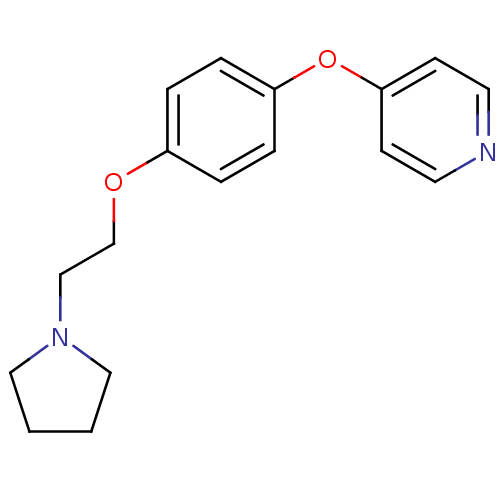 (4-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)pyridine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343918 (2-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)aniline | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343905 (4-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)pyridine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343925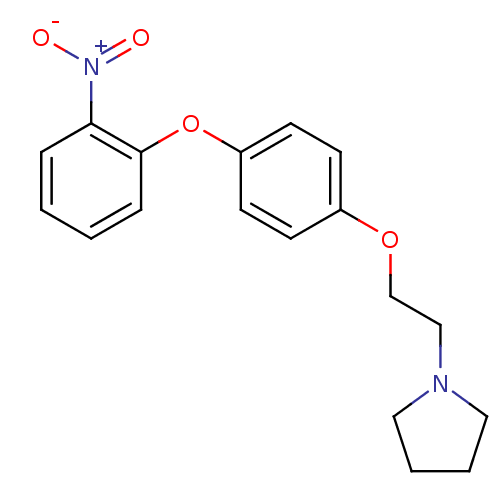 (1-(2-(4-(2-Nitrophenoxy)-phenoxy)ethyl)pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343919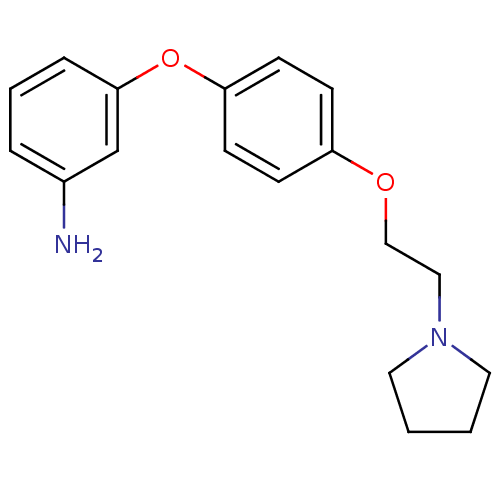 (3-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)aniline | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343927 (1-(2-(4-(4-Nitrophenoxy)phenoxy)ethyl)pyrrolidine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343916 (1-(2-(4-(4-Chlorophenoxy)phenoxy)ethyl)pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343912 (1-(2-(4-(2-(Trifluoromethyl)phenoxy)phenoxy)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343904 (2-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)pyridine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50085245 (1-[2-(4-Phenoxy-phenoxy)-ethyl]-pyrrolidine | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343919 (3-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)aniline | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343915 (1-(2-(4-(4-Fluorophenoxy)phenoxy)ethyl)pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343926 (1-(2-(4-(3-Nitrophenoxy)phenoxy)ethyl)pyrrolidine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50343920 (4-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)aniline | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of purified COX1 assessed as formation of oxidized TMPD during reduction og PGG2 to PGH2 preincubated for 15 mins by chromogenic assay | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343914 (1-(2-(4-(4-(Trifluoromethyl)phenoxy)phenoxy)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343923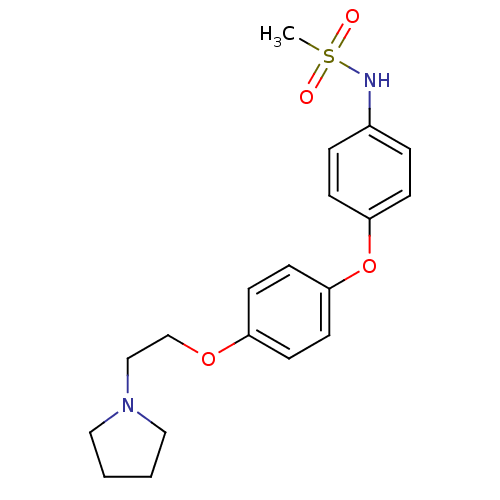 (CHEMBL1775110 | N-(4-(4-(2-(Pyrrolidin-1-yl)ethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343911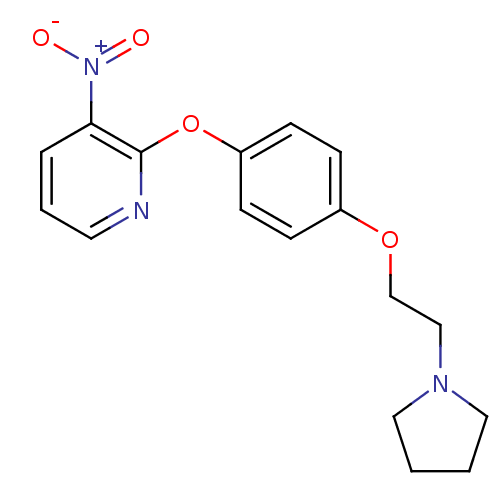 (3-Nitro-2-(4-(2-(pyrrolidin-1-yl)ethoxy)phenoxy)py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343923 (CHEMBL1775110 | N-(4-(4-(2-(Pyrrolidin-1-yl)ethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343910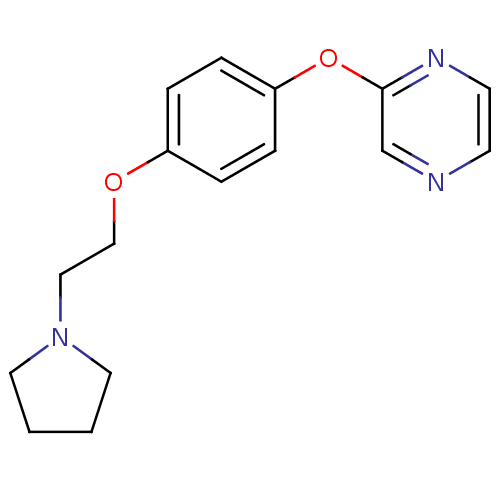 (2-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)pyrazine |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50343912 (1-(2-(4-(2-(Trifluoromethyl)phenoxy)phenoxy)ethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of LPS-induced PGE2 production after 24 hrs by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50343917 (1-(2-(4-(4-Bromophenoxy)phenoxy)ethyl)pyrrolidine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of LPS-induced PGE2 production after 24 hrs by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343925 (1-(2-(4-(2-Nitrophenoxy)-phenoxy)ethyl)pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50343925 (1-(2-(4-(2-Nitrophenoxy)-phenoxy)ethyl)pyrrolidine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of purified COX2 assessed as formation of oxidized TMPD during reduction og PGG2 to PGH2 preincubated for 15 mins by chromogenic assay | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50343925 (1-(2-(4-(2-Nitrophenoxy)-phenoxy)ethyl)pyrrolidine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of LPS-induced PGE2 production after 24 hrs by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50056999 (CHEMBL56367 | nimesulide) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of LPS-induced PGE2 production after 24 hrs by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50343926 (1-(2-(4-(3-Nitrophenoxy)phenoxy)ethyl)pyrrolidine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of LPS-induced PGE2 production after 24 hrs by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50343927 (1-(2-(4-(4-Nitrophenoxy)phenoxy)ethyl)pyrrolidine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of purified COX1 assessed as formation of oxidized TMPD during reduction og PGG2 to PGH2 preincubated for 15 mins by chromogenic assay | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50343916 (1-(2-(4-(4-Chlorophenoxy)phenoxy)ethyl)pyrrolidine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as inhibition of LPS-induced PGE2 production after 24 hrs by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50056999 (CHEMBL56367 | nimesulide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of purified COX1 assessed as formation of oxidized TMPD during reduction og PGG2 to PGH2 preincubated for 15 mins by chromogenic assay | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM50343911 (3-Nitro-2-(4-(2-(pyrrolidin-1-yl)ethoxy)phenoxy)py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of human LTA4 hydrolase activity assessed as LTB4 production after 15 mins by ELISA | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50343904 (2-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)pyridine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of purified COX2 assessed as formation of oxidized TMPD during reduction og PGG2 to PGH2 preincubated for 15 mins by chromogenic assay | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50343927 (1-(2-(4-(4-Nitrophenoxy)phenoxy)ethyl)pyrrolidine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of purified COX2 assessed as formation of oxidized TMPD during reduction og PGG2 to PGH2 preincubated for 15 mins by chromogenic assay | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50343920 (4-(4-(2-(Pyrrolidin-1-yl)ethoxy)phenoxy)aniline | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of purified COX2 assessed as formation of oxidized TMPD during reduction og PGG2 to PGH2 preincubated for 15 mins by chromogenic assay | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50343912 (1-(2-(4-(2-(Trifluoromethyl)phenoxy)phenoxy)ethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of purified COX2 assessed as formation of oxidized TMPD during reduction og PGG2 to PGH2 preincubated for 15 mins by chromogenic assay | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50343925 (1-(2-(4-(2-Nitrophenoxy)-phenoxy)ethyl)pyrrolidine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of purified COX1 assessed as formation of oxidized TMPD during reduction og PGG2 to PGH2 preincubated for 15 mins by chromogenic assay | J Med Chem 54: 3650-60 (2011) Article DOI: 10.1021/jm200063s BindingDB Entry DOI: 10.7270/Q24M94WS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 135 total ) | Next | Last >> |