 Found 28 hits Enz. Inhib. hit(s) with all data for entry = 50034523
Found 28 hits Enz. Inhib. hit(s) with all data for entry = 50034523 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50043280

(4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1H-benzimidaz...)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)-c1nc2ccccc2n1C Show InChI InChI=1S/C33H30N4O2/c1-4-9-30-35-31-21(2)18-24(32-34-27-12-7-8-13-28(27)36(32)3)19-29(31)37(30)20-22-14-16-23(17-15-22)25-10-5-6-11-26(25)33(38)39/h5-8,10-19H,4,9,20H2,1-3H3,(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363833

(CHEMBL1945010)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1cc(OC)ccc1OC Show InChI InChI=1S/C36H37N3O5/c1-5-8-33-38-34-23(2)19-27(35(40)37-18-17-26-20-28(43-3)15-16-32(26)44-4)21-31(34)39(33)22-24-11-13-25(14-12-24)29-9-6-7-10-30(29)36(41)42/h6-7,9-16,19-21H,5,8,17-18,22H2,1-4H3,(H,37,40)(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363826
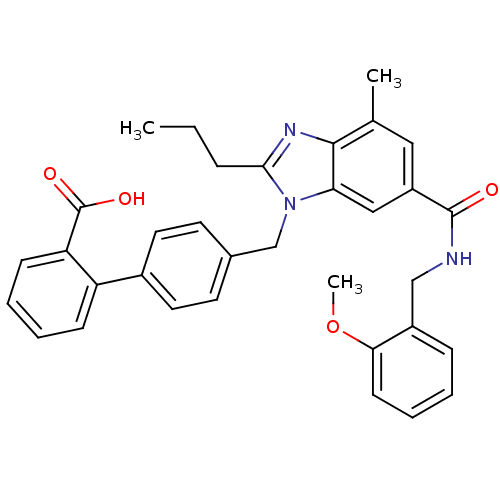
(CHEMBL1947129)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccccc1OC Show InChI InChI=1S/C34H33N3O4/c1-4-9-31-36-32-22(2)18-26(33(38)35-20-25-10-5-8-13-30(25)41-3)19-29(32)37(31)21-23-14-16-24(17-15-23)27-11-6-7-12-28(27)34(39)40/h5-8,10-19H,4,9,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363828
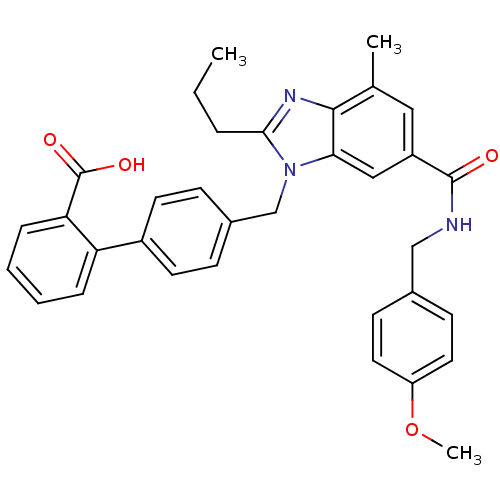
(CHEMBL1947131)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccc(OC)cc1 Show InChI InChI=1S/C34H33N3O4/c1-4-7-31-36-32-22(2)18-26(33(38)35-20-23-12-16-27(41-3)17-13-23)19-30(32)37(31)21-24-10-14-25(15-11-24)28-8-5-6-9-29(28)34(39)40/h5-6,8-19H,4,7,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363829
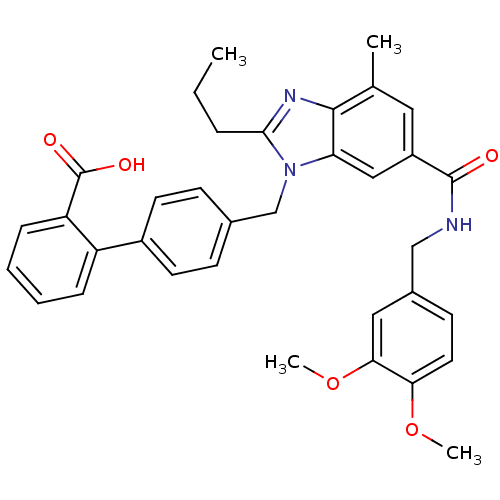
(CHEMBL1945006)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccc(OC)c(OC)c1 Show InChI InChI=1S/C35H35N3O5/c1-5-8-32-37-33-22(2)17-26(34(39)36-20-24-13-16-30(42-3)31(18-24)43-4)19-29(33)38(32)21-23-11-14-25(15-12-23)27-9-6-7-10-28(27)35(40)41/h6-7,9-19H,5,8,20-21H2,1-4H3,(H,36,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363830
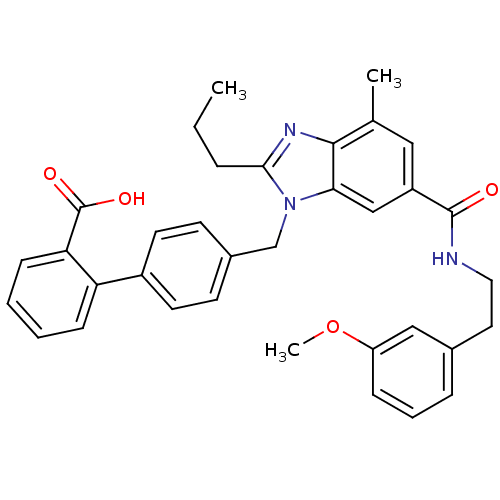
(CHEMBL1945007)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1cccc(OC)c1 Show InChI InChI=1S/C35H35N3O4/c1-4-8-32-37-33-23(2)19-27(34(39)36-18-17-24-9-7-10-28(20-24)42-3)21-31(33)38(32)22-25-13-15-26(16-14-25)29-11-5-6-12-30(29)35(40)41/h5-7,9-16,19-21H,4,8,17-18,22H2,1-3H3,(H,36,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363825

(CHEMBL1947128)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C33H31N3O3/c1-3-9-30-35-31-22(2)18-26(32(37)34-20-23-10-5-4-6-11-23)19-29(31)36(30)21-24-14-16-25(17-15-24)27-12-7-8-13-28(27)33(38)39/h4-8,10-19H,3,9,20-21H2,1-2H3,(H,34,37)(H,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363832

(CHEMBL1945009)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1ccc(OC)c(OC)c1 Show InChI InChI=1S/C36H37N3O5/c1-5-8-33-38-34-23(2)19-27(35(40)37-18-17-24-13-16-31(43-3)32(20-24)44-4)21-30(34)39(33)22-25-11-14-26(15-12-25)28-9-6-7-10-29(28)36(41)42/h6-7,9-16,19-21H,5,8,17-18,22H2,1-4H3,(H,37,40)(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363834

(CHEMBL1945148)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1ccccc1F Show InChI InChI=1S/C34H32FN3O3/c1-3-8-31-37-32-22(2)19-26(33(39)36-18-17-25-9-4-7-12-29(25)35)20-30(32)38(31)21-23-13-15-24(16-14-23)27-10-5-6-11-28(27)34(40)41/h4-7,9-16,19-20H,3,8,17-18,21H2,1-2H3,(H,36,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363835

(CHEMBL1945149)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1ccc(F)cc1 Show InChI InChI=1S/C34H32FN3O3/c1-3-6-31-37-32-22(2)19-26(33(39)36-18-17-23-11-15-27(35)16-12-23)20-30(32)38(31)21-24-9-13-25(14-10-24)28-7-4-5-8-29(28)34(40)41/h4-5,7-16,19-20H,3,6,17-18,21H2,1-2H3,(H,36,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363831

(CHEMBL1945008)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1ccc(OC)cc1 Show InChI InChI=1S/C35H35N3O4/c1-4-7-32-37-33-23(2)20-27(34(39)36-19-18-24-12-16-28(42-3)17-13-24)21-31(33)38(32)22-25-10-14-26(15-11-25)29-8-5-6-9-30(29)35(40)41/h5-6,8-17,20-21H,4,7,18-19,22H2,1-3H3,(H,36,39)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363827
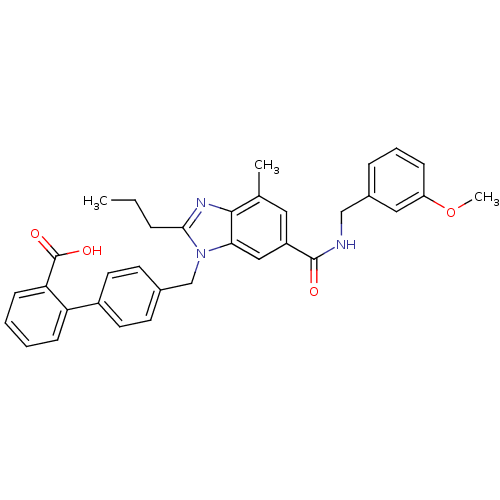
(CHEMBL1947130)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1cccc(OC)c1 Show InChI InChI=1S/C34H33N3O4/c1-4-8-31-36-32-22(2)17-26(33(38)35-20-24-9-7-10-27(18-24)41-3)19-30(32)37(31)21-23-13-15-25(16-14-23)28-11-5-6-12-29(28)34(39)40/h5-7,9-19H,4,8,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 326 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT1 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363826

(CHEMBL1947129)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccccc1OC Show InChI InChI=1S/C34H33N3O4/c1-4-9-31-36-32-22(2)18-26(33(38)35-20-25-10-5-8-13-30(25)41-3)19-29(32)37(31)21-23-14-16-24(17-15-23)27-11-6-7-12-28(27)34(39)40/h5-8,10-19H,4,9,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363832

(CHEMBL1945009)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1ccc(OC)c(OC)c1 Show InChI InChI=1S/C36H37N3O5/c1-5-8-33-38-34-23(2)19-27(35(40)37-18-17-24-13-16-31(43-3)32(20-24)44-4)21-30(34)39(33)22-25-11-14-26(15-12-25)28-9-6-7-10-29(28)36(41)42/h6-7,9-16,19-21H,5,8,17-18,22H2,1-4H3,(H,37,40)(H,41,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363835

(CHEMBL1945149)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1ccc(F)cc1 Show InChI InChI=1S/C34H32FN3O3/c1-3-6-31-37-32-22(2)19-26(33(39)36-18-17-23-11-15-27(35)16-12-23)20-30(32)38(31)21-24-9-13-25(14-10-24)28-7-4-5-8-29(28)34(40)41/h4-5,7-16,19-20H,3,6,17-18,21H2,1-2H3,(H,36,39)(H,40,41) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363833

(CHEMBL1945010)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1cc(OC)ccc1OC Show InChI InChI=1S/C36H37N3O5/c1-5-8-33-38-34-23(2)19-27(35(40)37-18-17-26-20-28(43-3)15-16-32(26)44-4)21-31(34)39(33)22-24-11-13-25(14-12-24)29-9-6-7-10-30(29)36(41)42/h6-7,9-16,19-21H,5,8,17-18,22H2,1-4H3,(H,37,40)(H,41,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363831

(CHEMBL1945008)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1ccc(OC)cc1 Show InChI InChI=1S/C35H35N3O4/c1-4-7-32-37-33-23(2)20-27(34(39)36-19-18-24-12-16-28(42-3)17-13-24)21-31(33)38(32)22-25-10-14-26(15-11-25)29-8-5-6-9-30(29)35(40)41/h5-6,8-17,20-21H,4,7,18-19,22H2,1-3H3,(H,36,39)(H,40,41) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363828

(CHEMBL1947131)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccc(OC)cc1 Show InChI InChI=1S/C34H33N3O4/c1-4-7-31-36-32-22(2)18-26(33(38)35-20-23-12-16-27(41-3)17-13-23)19-30(32)37(31)21-24-10-14-25(15-11-24)28-8-5-6-9-29(28)34(39)40/h5-6,8-19H,4,7,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363829

(CHEMBL1945006)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccc(OC)c(OC)c1 Show InChI InChI=1S/C35H35N3O5/c1-5-8-32-37-33-22(2)17-26(34(39)36-20-24-13-16-30(42-3)31(18-24)43-4)19-29(33)38(32)21-23-11-14-25(15-12-23)27-9-6-7-10-28(27)35(40)41/h6-7,9-19H,5,8,20-21H2,1-4H3,(H,36,39)(H,40,41) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363827

(CHEMBL1947130)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1cccc(OC)c1 Show InChI InChI=1S/C34H33N3O4/c1-4-8-31-36-32-22(2)17-26(33(38)35-20-24-9-7-10-27(18-24)41-3)19-30(32)37(31)21-23-13-15-25(16-14-23)28-11-5-6-12-29(28)34(39)40/h5-7,9-19H,4,8,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50363830

(CHEMBL1945007)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1cccc(OC)c1 Show InChI InChI=1S/C35H35N3O4/c1-4-8-32-37-33-23(2)19-27(34(39)36-18-17-24-9-7-10-28(20-24)42-3)21-31(33)38(32)22-25-13-15-26(16-14-25)29-11-5-6-12-30(29)35(40)41/h5-7,9-16,19-21H,4,8,17-18,22H2,1-3H3,(H,36,39)(H,40,41) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Displacement of [125I]Sar1 Ile8-Ang 2 from angiotensin 2 AT2 receptor after 180 mins by gamma counting |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM82258

(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 AT1 receptor in Japanese White rabbits thoracic aorta assessed as inhibition of KCl-indcuced contraction after 6... |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50363826

(CHEMBL1947129)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccccc1OC Show InChI InChI=1S/C34H33N3O4/c1-4-9-31-36-32-22(2)18-26(33(38)35-20-25-10-5-8-13-30(25)41-3)19-29(32)37(31)21-23-14-16-24(17-15-23)27-11-6-7-12-28(27)34(39)40/h5-8,10-19H,4,9,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.92 | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 AT1 receptor in Japanese White rabbits thoracic aorta assessed as inhibition of KCl-indcuced contraction after 6... |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50363828

(CHEMBL1947131)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccc(OC)cc1 Show InChI InChI=1S/C34H33N3O4/c1-4-7-31-36-32-22(2)18-26(33(38)35-20-23-12-16-27(41-3)17-13-23)19-30(32)37(31)21-24-10-14-25(15-11-24)28-8-5-6-9-29(28)34(39)40/h5-6,8-19H,4,7,20-21H2,1-3H3,(H,35,38)(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.79 | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 AT1 receptor in Japanese White rabbits thoracic aorta assessed as inhibition of KCl-indcuced contraction after 6... |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(RABBIT) | BDBM50363833

(CHEMBL1945010)Show SMILES CCCc1nc2c(C)cc(cc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCCc1cc(OC)ccc1OC Show InChI InChI=1S/C36H37N3O5/c1-5-8-33-38-34-23(2)19-27(35(40)37-18-17-26-20-28(43-3)15-16-32(26)44-4)21-31(34)39(33)22-24-11-13-25(14-12-24)29-9-6-7-10-30(29)36(41)42/h6-7,9-16,19-21H,5,8,17-18,22H2,1-4H3,(H,37,40)(H,41,42) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a |
Beijing Institute of Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at angiotensin 2 AT1 receptor in Japanese White rabbits thoracic aorta assessed as inhibition of KCl-indcuced contraction after 6... |
Eur J Med Chem 49: 183-90 (2012)
Article DOI: 10.1016/j.ejmech.2012.01.009
BindingDB Entry DOI: 10.7270/Q29K4BPW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data