

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403242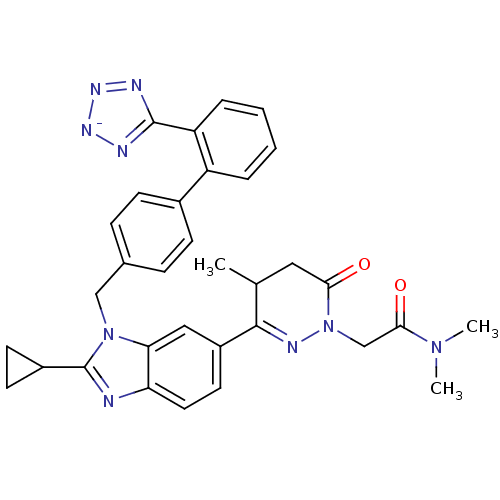 (CHEMBL2079790) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403247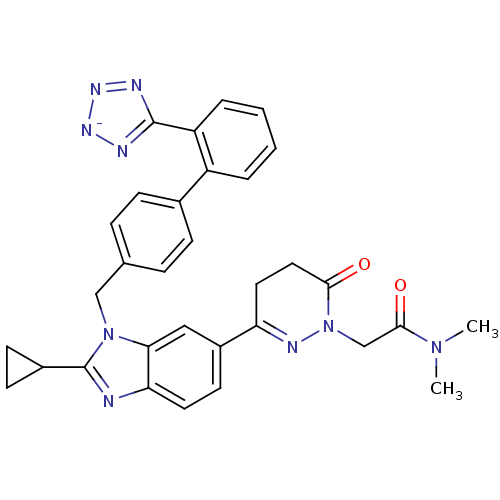 (CHEMBL2079791) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282613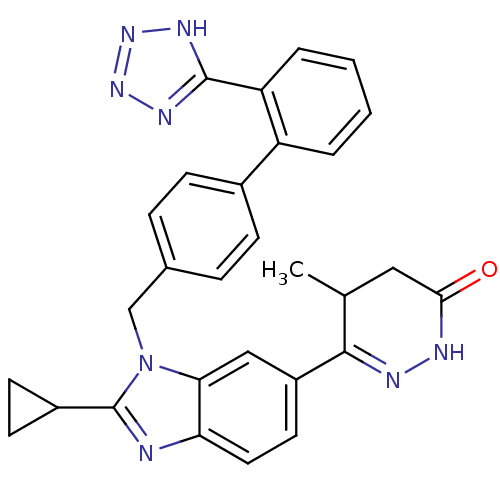 (6-{2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403246 (CHEMBL2079787) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403245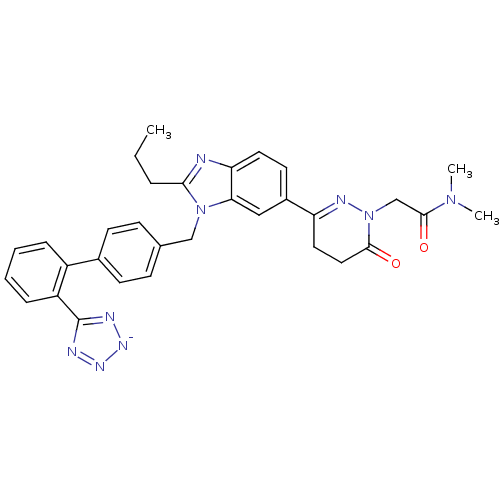 (CHEMBL2079788) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282616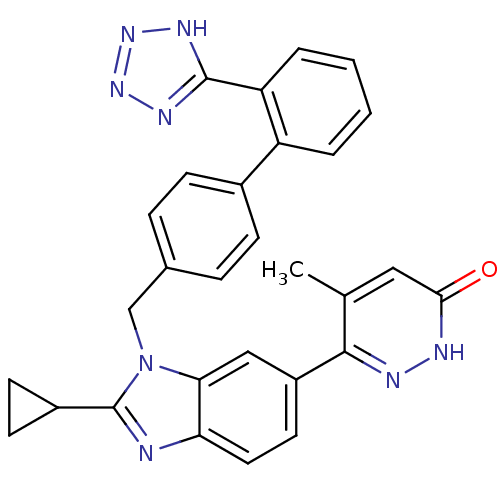 (6-{2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403243 (CHEMBL2079789) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50282611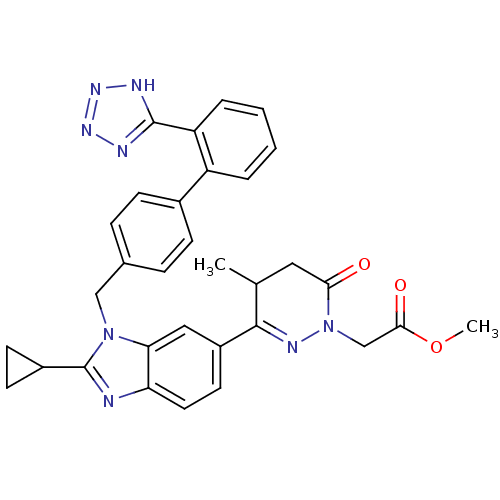 ((3-{2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-bipheny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50403244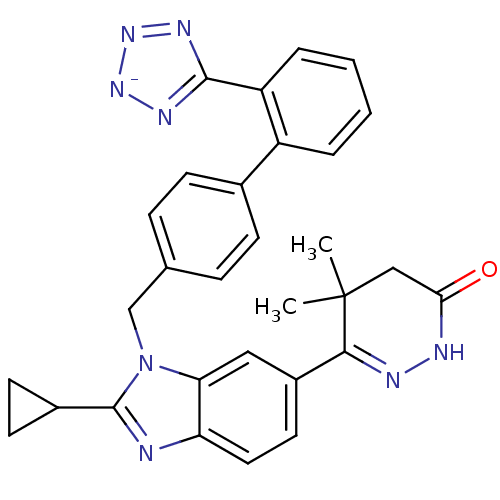 (CHEMBL2079785) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 induced contractions in isolated rabbit aortic rings | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282626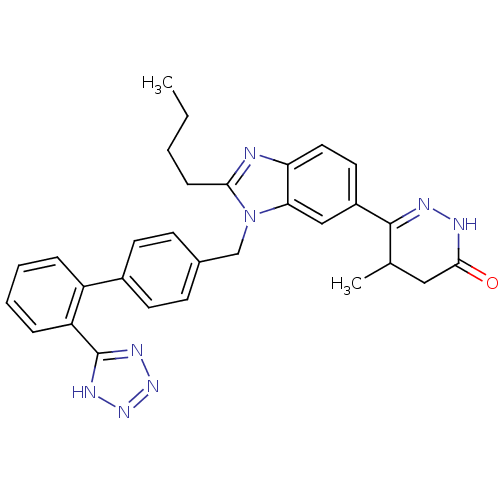 (6-{2-Butyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of binding against Angiotensin II receptor, type 1 in bovine adrenal cortex | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282629 (6-{2-Butyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of binding against Angiotensin II receptor, type 1 in bovine adrenal cortex | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282623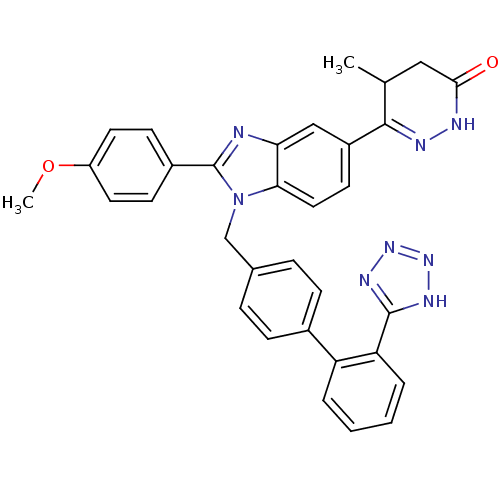 (6-{2-(4-Methoxy-phenyl)-1-[2'-(1H-tetrazol-5-yl)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of binding against Angiotensin II receptor, type 1 in bovine adrenal cortex | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282621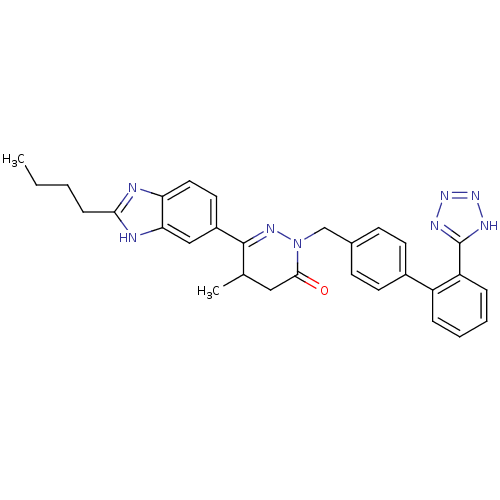 (6-(2-Butyl-1H-benzoimidazol-5-yl)-5-methyl-2-[2'-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of binding against Angiotensin II receptor, type 1 in bovine adrenal cortex | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282617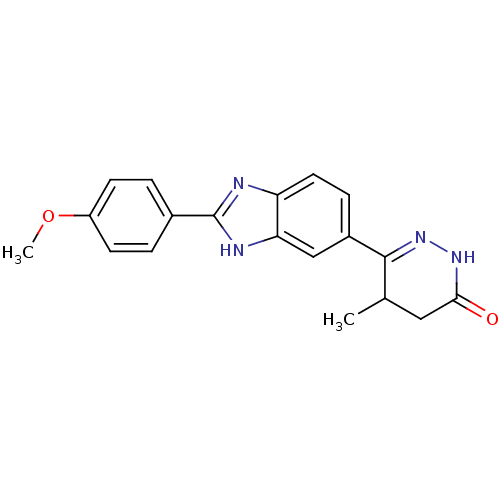 (6-[2-(4-methoxyphenyl)-1H-benzimidazol-5-yl]-5-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of binding against Angiotensin II receptor, type 1 in bovine adrenal cortex | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282615 (6-[2-(4-Methoxy-phenyl)-1H-benzoimidazol-5-yl]-5-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of binding against Angiotensin II receptor, type 1 in bovine adrenal cortex | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Bos taurus) | BDBM50282612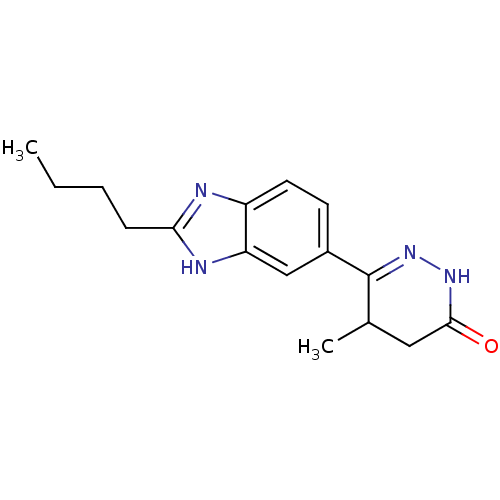 (6-(2-Butyl-1H-benzoimidazol-5-yl)-5-methyl-4,5-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article | n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of binding against Angiotensin II receptor, type 1 in bovine adrenal cortex | Bioorg Med Chem Lett 4: 1297-1302 (1994) Article DOI: 10.1016/S0960-894X(01)80348-6 BindingDB Entry DOI: 10.7270/Q2G44QRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||