 Found 30 hits Enz. Inhib. hit(s) with all data for entry = 50035418
Found 30 hits Enz. Inhib. hit(s) with all data for entry = 50035418 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Caspase-9
(Homo sapiens (Human)) | BDBM50140543
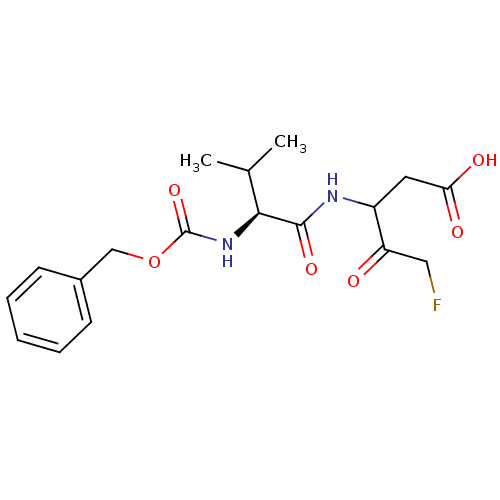
(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Caspase-9 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-8
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Caspase-8 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-7
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Caspase-7 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-6
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Caspase-6 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Caspase-1 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50366934

(CHEMBL1794026)Show SMILES CC[C@H](C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-3-12(2)17(18(26)21-14(9-16(24)25)15(23)10-20)22-19(27)28-11-13-7-5-4-6-8-13/h4-8,12,14,17H,3,9-11H2,1-2H3,(H,21,26)(H,22,27)(H,24,25)/t12-,14?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140549

(3-((S)-2-Benzyloxycarbonylamino-2-cyclohexyl-acety...)Show SMILES OC(=O)CC(NC(=O)[C@@H](NC(=O)OCc1ccccc1)C1CCCCC1)C(=O)CF Show InChI InChI=1S/C21H27FN2O6/c22-12-17(25)16(11-18(26)27)23-20(28)19(15-9-5-2-6-10-15)24-21(29)30-13-14-7-3-1-4-8-14/h1,3-4,7-8,15-16,19H,2,5-6,9-13H2,(H,23,28)(H,24,29)(H,26,27)/t16?,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140551

(3-((S)-2-Benzyloxycarbonylamino-2-phenyl-acetylami...)Show SMILES OC(=O)CC(NC(=O)[C@@H](NC(=O)OCc1ccccc1)c1ccccc1)C(=O)CF Show InChI InChI=1S/C21H21FN2O6/c22-12-17(25)16(11-18(26)27)23-20(28)19(15-9-5-2-6-10-15)24-21(29)30-13-14-7-3-1-4-8-14/h1-10,16,19H,11-13H2,(H,23,28)(H,24,29)(H,26,27)/t16?,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140552
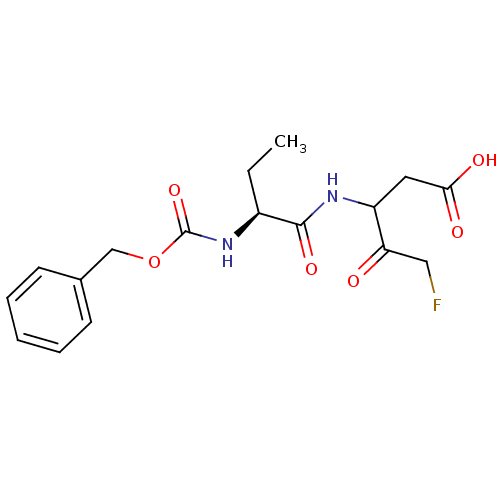
(3-((S)-2-Benzyloxycarbonylamino-butyrylamino)-5-fl...)Show SMILES CC[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C17H21FN2O6/c1-2-12(16(24)19-13(8-15(22)23)14(21)9-18)20-17(25)26-10-11-6-4-3-5-7-11/h3-7,12-13H,2,8-10H2,1H3,(H,19,24)(H,20,25)(H,22,23)/t12-,13?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50140548

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES COC(=O)CC(NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-12(2)17(22-19(26)28-11-13-7-5-4-6-8-13)18(25)21-14(15(23)10-20)9-16(24)27-3/h4-8,12,14,17H,9-11H2,1-3H3,(H,21,25)(H,22,26)/t14?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Calpain-1 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140550

(3-((S)-2-Benzyloxycarbonylamino-3-thiophen-2-yl-pr...)Show SMILES OC(=O)CC(NC(=O)[C@H](Cc1cccs1)NC(=O)OCc1ccccc1)C(=O)CF Show InChI InChI=1S/C20H21FN2O6S/c21-11-17(24)15(10-18(25)26)22-19(27)16(9-14-7-4-8-30-14)23-20(28)29-12-13-5-2-1-3-6-13/h1-8,15-16H,9-12H2,(H,22,27)(H,23,28)(H,25,26)/t15?,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140544
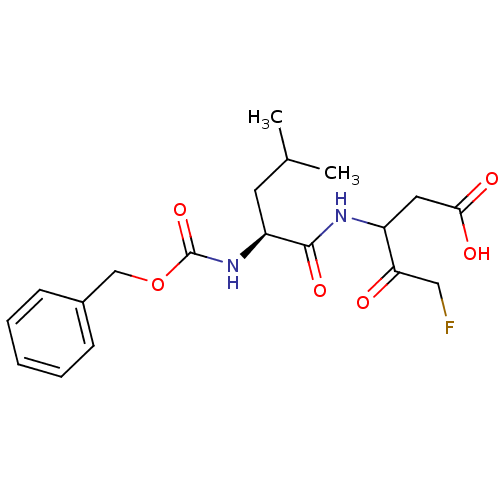
(3-((S)-2-Benzyloxycarbonylamino-4-methyl-pentanoyl...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-12(2)8-15(18(26)21-14(9-17(24)25)16(23)10-20)22-19(27)28-11-13-6-4-3-5-7-13/h3-7,12,14-15H,8-11H2,1-2H3,(H,21,26)(H,22,27)(H,24,25)/t14?,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50140548

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES COC(=O)CC(NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-12(2)17(22-19(26)28-11-13-7-5-4-6-8-13)18(25)21-14(15(23)10-20)9-16(24)27-3/h4-8,12,14,17H,9-11H2,1-3H3,(H,21,25)(H,22,26)/t14?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against Cathepsin B |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140558

(3-((S)-2-Benzyloxycarbonylamino-3-phenyl-propionyl...)Show SMILES OC(=O)CC(NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(=O)CF Show InChI InChI=1S/C22H23FN2O6/c23-13-19(26)17(12-20(27)28)24-21(29)18(11-15-7-3-1-4-8-15)25-22(30)31-14-16-9-5-2-6-10-16/h1-10,17-18H,11-14H2,(H,24,29)(H,25,30)(H,27,28)/t17?,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140546
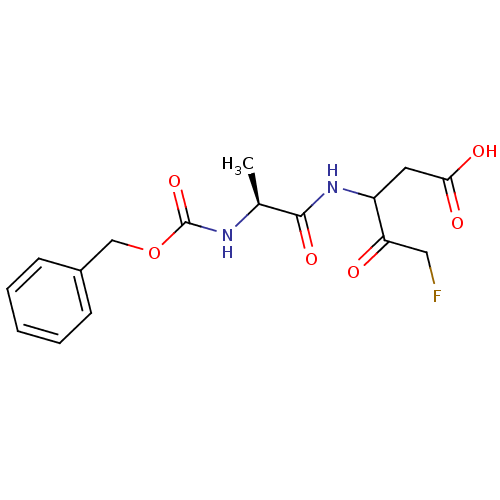
(3-((S)-2-Benzyloxycarbonylamino-propionylamino)-5-...)Show SMILES C[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C16H19FN2O6/c1-10(15(23)19-12(7-14(21)22)13(20)8-17)18-16(24)25-9-11-5-3-2-4-6-11/h2-6,10,12H,7-9H2,1H3,(H,18,24)(H,19,23)(H,21,22)/t10-,12?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50140548

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES COC(=O)CC(NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-12(2)17(22-19(26)28-11-13-7-5-4-6-8-13)18(25)21-14(15(23)10-20)9-16(24)27-3/h4-8,12,14,17H,9-11H2,1-3H3,(H,21,25)(H,22,26)/t14?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Caspase-1 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140548

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES COC(=O)CC(NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-12(2)17(22-19(26)28-11-13-7-5-4-6-8-13)18(25)21-14(15(23)10-20)9-16(24)27-3/h4-8,12,14,17H,9-11H2,1-3H3,(H,21,25)(H,22,26)/t14?,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140553
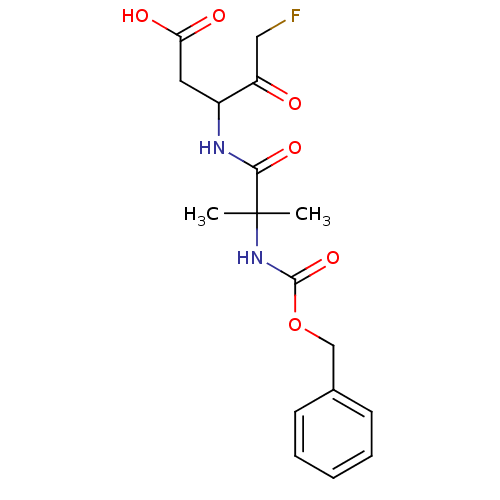
(3-(2-Benzyloxycarbonylamino-2-methyl-propionylamin...)Show SMILES CC(C)(NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C17H21FN2O6/c1-17(2,15(24)19-12(8-14(22)23)13(21)9-18)20-16(25)26-10-11-6-4-3-5-7-11/h3-7,12H,8-10H2,1-2H3,(H,19,24)(H,20,25)(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140557

(3-((S)-6-Amino-2-benzyloxycarbonylamino-hexanoylam...)Show SMILES NCCCC[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C19H26FN3O6/c20-11-16(24)15(10-17(25)26)22-18(27)14(8-4-5-9-21)23-19(28)29-12-13-6-2-1-3-7-13/h1-3,6-7,14-15H,4-5,8-12,21H2,(H,22,27)(H,23,28)(H,25,26)/t14-,15?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140556
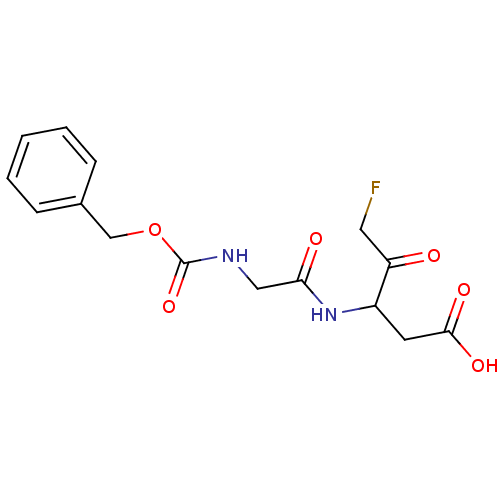
(3-(2-Benzyloxycarbonylamino-acetylamino)-5-fluoro-...)Show InChI InChI=1S/C15H17FN2O6/c16-7-12(19)11(6-14(21)22)18-13(20)8-17-15(23)24-9-10-4-2-1-3-5-10/h1-5,11H,6-9H2,(H,17,23)(H,18,20)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140554

(3-(2-Benzyloxycarbonylamino-2,3-dimethyl-butyrylam...)Show SMILES CC(C)C(C)(NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-12(2)19(3,17(26)21-14(9-16(24)25)15(23)10-20)22-18(27)28-11-13-7-5-4-6-8-13/h4-8,12,14H,9-11H2,1-3H3,(H,21,26)(H,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50366933

(CHEMBL1794025)Show SMILES CC[C@H](C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(=O)OC)C(=O)CF Show InChI InChI=1S/C20H27FN2O6/c1-4-13(2)18(23-20(27)29-12-14-8-6-5-7-9-14)19(26)22-15(16(24)11-21)10-17(25)28-3/h5-9,13,15,18H,4,10-12H2,1-3H3,(H,22,26)(H,23,27)/t13-,15?,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Calpain 1 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against Cathepsin B |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50140555
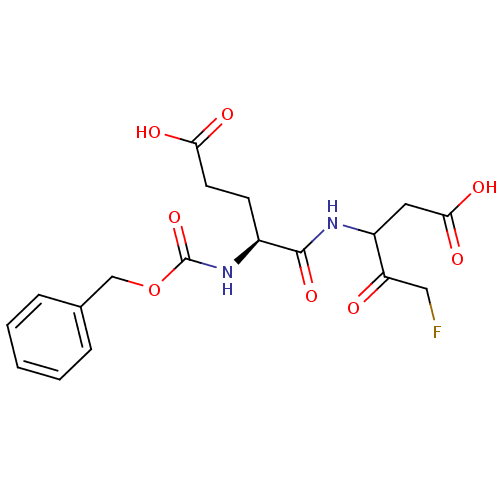
(3-((S)-2-Benzyloxycarbonylamino-4-carboxy-butyryla...)Show SMILES OC(=O)CC[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H21FN2O8/c19-9-14(22)13(8-16(25)26)20-17(27)12(6-7-15(23)24)21-18(28)29-10-11-4-2-1-3-5-11/h1-5,12-13H,6-10H2,(H,20,27)(H,21,28)(H,23,24)(H,25,26)/t12-,13?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human recombinant Caspase-3 enzyme |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50140548

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES COC(=O)CC(NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-12(2)17(22-19(26)28-11-13-7-5-4-6-8-13)18(25)21-14(15(23)10-20)9-16(24)27-3/h4-8,12,14,17H,9-11H2,1-3H3,(H,21,25)(H,22,26)/t14?,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Thrombin |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Coagulation factor X |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50140543

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(CC(O)=O)C(=O)CF Show InChI InChI=1S/C18H23FN2O6/c1-11(2)16(17(25)20-13(8-15(23)24)14(22)9-19)21-18(26)27-10-12-6-4-3-5-7-12/h3-7,11,13,16H,8-10H2,1-2H3,(H,20,25)(H,21,26)(H,23,24)/t13?,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Thrombin |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50140548

(3-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylam...)Show SMILES COC(=O)CC(NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C)C(=O)CF Show InChI InChI=1S/C19H25FN2O6/c1-12(2)17(22-19(26)28-11-13-7-5-4-6-8-13)18(25)21-14(15(23)10-20)9-16(24)27-3/h4-8,12,14,17H,9-11H2,1-3H3,(H,21,25)(H,22,26)/t14?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Maxim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against the Coagulation factor X |
Bioorg Med Chem Lett 14: 1269-72 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.065
BindingDB Entry DOI: 10.7270/Q2V988NB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data