 Found 14 hits Enz. Inhib. hit(s) with all data for entry = 50040555
Found 14 hits Enz. Inhib. hit(s) with all data for entry = 50040555 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50083164

((S)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NC12C[C@H]3C[C@H](C[C@H](C3)C1)C2 |TLB:30:31:35:29.28.34,THB:30:29:35:31.36.32| Show InChI InChI=1S/C31H39N3O3/c1-18-7-25(35)8-19(2)26(18)13-27(32)30(37)34-17-24-6-4-3-5-23(24)12-28(34)29(36)33-31-14-20-9-21(15-31)11-22(10-20)16-31/h3-8,20-22,27-28,35H,9-17,32H2,1-2H3,(H,33,36)/t20-,21+,22-,27-,28-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at DOR |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50323341
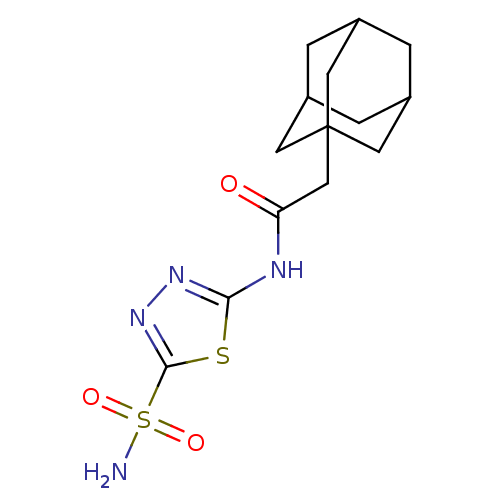
(CHEMBL1209039)Show SMILES NS(=O)(=O)c1nnc(NC(=O)CC23CC4CC(CC(C4)C2)C3)s1 |TLB:11:12:15:19.18.17,THB:13:14:17:21.12.20,13:12:15.14.19:17,20:12:15:19.18.17,20:18:15:21.13.12| Show InChI InChI=1S/C14H20N4O3S2/c15-23(20,21)13-18-17-12(22-13)16-11(19)7-14-4-8-1-9(5-14)3-10(2-8)6-14/h8-10H,1-7H2,(H2,15,20,21)(H,16,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50396058

(CHEMBL2170198)Show SMILES NS(=O)(=O)c1nnc(NC(=O)OC23CC4CC(CC(C4)C2)C3)s1 |TLB:11:12:15:19.17.18,THB:17:16:13:19.18.20,17:18:15.16.21:13,20:18:15:21.12.13,20:12:15:19.17.18| Show InChI InChI=1S/C13H18N4O4S2/c14-23(19,20)12-17-16-10(22-12)15-11(18)21-13-4-7-1-8(5-13)3-9(2-7)6-13/h7-9H,1-6H2,(H2,14,19,20)(H,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM82104
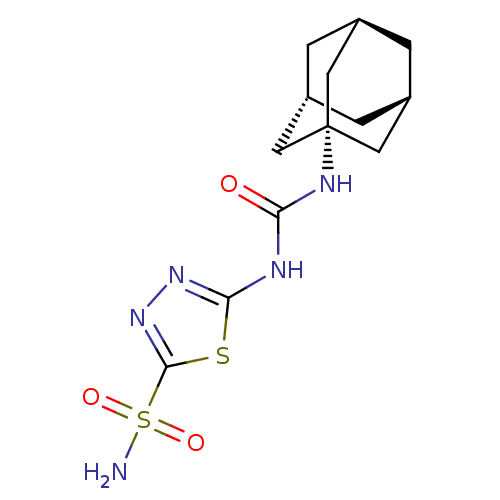
(Investigational agent, 5)Show SMILES NS(=O)(=O)c1nnc(NC(=O)N[C@]23C[C@H]4C[C@H](C[C@H](C4)C2)C3)s1 |r,THB:13:14:20.12.21:17,15:14:20:16.21.17| Show InChI InChI=1S/C13H19N5O3S2/c14-23(20,21)12-18-17-11(22-12)15-10(19)16-13-4-7-1-8(5-13)3-9(2-7)6-13/h7-9H,1-6H2,(H2,14,20,21)(H2,15,16,17,19)/t7-,8+,9-,13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50083164

((S)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...)Show SMILES Cc1cc(O)cc(C)c1C[C@H](N)C(=O)N1Cc2ccccc2C[C@H]1C(=O)NC12C[C@H]3C[C@H](C[C@H](C3)C1)C2 |TLB:30:31:35:29.28.34,THB:30:29:35:31.36.32| Show InChI InChI=1S/C31H39N3O3/c1-18-7-25(35)8-19(2)26(18)13-27(32)30(37)34-17-24-6-4-3-5-23(24)12-28(34)29(36)33-31-14-20-9-21(15-31)11-22(10-20)16-31/h3-8,20-22,27-28,35H,9-17,32H2,1-2H3,(H,33,36)/t20-,21+,22-,27-,28-,31?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Antagonist activity at MOR |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50299749

((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:19.20.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:19:27.21.22| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20-,21-,22?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of non-lysosomal glucosylceramidase in cultured melanoma cells |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50299749

((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:19.20.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:19:27.21.22| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20-,21-,22?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of non-lysosomal glucosylceramidase |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50396056
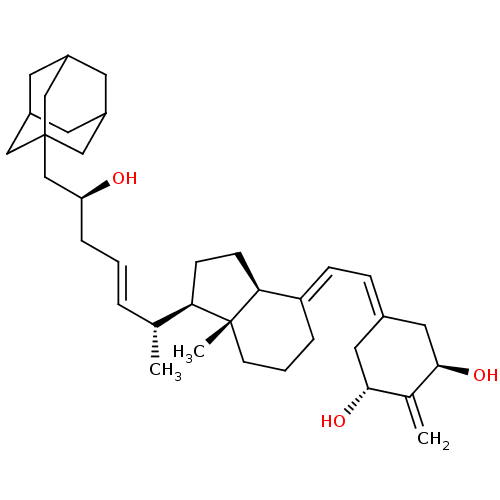
(CHEMBL2170202)Show SMILES [#6]-[#6@H](\[#6]=[#6]\[#6]-[#6@H](-[#8])-[#6]C12[#6]-[#6]-3-[#6]-[#6](-[#6]-[#6](-[#6]-3)-[#6]1)-[#6]2)-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r,TLB:7:8:11:15.13.14,THB:13:12:9:15.14.16,13:14:11.12.17:9,16:14:11:17.8.9,16:8:11:15.13.14| Show InChI InChI=1S/C36H54O3/c1-23(6-4-8-30(37)22-36-19-26-14-27(20-36)16-28(15-26)21-36)31-11-12-32-29(7-5-13-35(31,32)3)10-9-25-17-33(38)24(2)34(39)18-25/h4,6,9-10,23,26-28,30-34,37-39H,2,5,7-8,11-22H2,1,3H3/b6-4+,29-10+/t23-,26?,27?,28?,30+,31-,32+,33-,34-,35-,36?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Transactivation of VDR expressed in COS7 cells assessed as reduction in 1,25-dihydroxyvitamin D3-induced transcriptional activity by transient transc... |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant soluble epoxide hydrolase |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant soluble epoxide hydrolase |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM18355

((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...)Show InChI InChI=1S/C10H21NO4/c1-2-3-4-11-5-8(13)10(15)9(14)7(11)6-12/h7-10,12-15H,2-6H2,1H3/t7-,8+,9-,10-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of non-lysosomal glucosylceramidase in cultured melanoma cells |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of non-lysosomal glucosylceramidase in cultured melanoma cells |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |
Mediator of RNA polymerase II transcription subunit 23
(Homo sapiens (Human)) | BDBM50396057
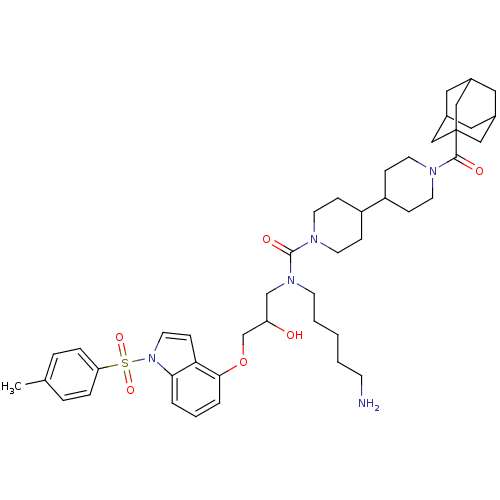
(CHEMBL2170196)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1ccc2c(OCC(O)CN(CCCCCN)C(=O)N3CCC(CC3)C3CCN(CC3)C(=O)C34CC5CC(CC(C5)C3)C4)cccc12 |TLB:41:43:46:50.48.49,THB:48:47:44:50.49.51,48:49:46.47.52:44,51:49:46:52.43.44,51:43:46:50.48.49| Show InChI InChI=1S/C45H63N5O6S/c1-32-8-10-39(11-9-32)57(54,55)50-23-16-40-41(50)6-5-7-42(40)56-31-38(51)30-49(18-4-2-3-17-46)44(53)48-21-14-37(15-22-48)36-12-19-47(20-13-36)43(52)45-27-33-24-34(28-45)26-35(25-33)29-45/h5-11,16,23,33-38,51H,2-4,12-15,17-22,24-31,46H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a |
University of Sydney
Curated by ChEMBL
| Assay Description
Binding affinity to Sur2 |
Eur J Med Chem 46: 1949-63 (2011)
Article DOI: 10.1016/j.ejmech.2011.01.047
BindingDB Entry DOI: 10.7270/Q22808QQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data