 Found 30 hits Enz. Inhib. hit(s) with all data for entry = 50043115
Found 30 hits Enz. Inhib. hit(s) with all data for entry = 50043115 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bile acid receptor
(Homo sapiens (Human)) | BDBM50436203
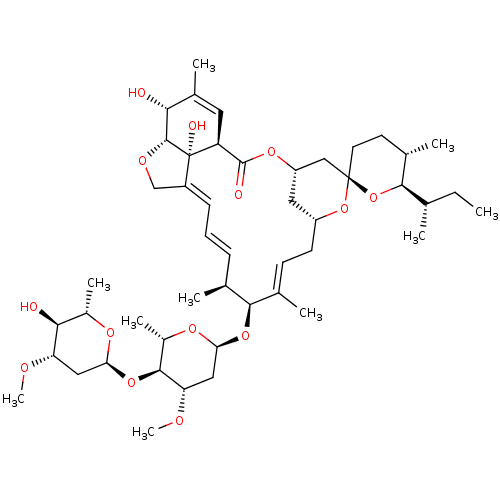
(CHEMBL3039587)Show SMILES CC[C@H](C)[C@H]1O[C@]2(CC[C@@H]1C)C[C@@H]1C[C@@H](C\C=C(C)\[C@@H](O[C@H]3C[C@H](OC)[C@@H](O[C@H]4C[C@H](OC)[C@@H](O)[C@H](C)O4)[C@H](C)O3)[C@@H](C)\C=C\C=C3/CO[C@@H]4[C@H](O)C(C)=C[C@@H](C(=O)O1)[C@@]34O)O2 |r,c:17,48,56,t:46| Show InChI InChI=1S/C48H74O14/c1-11-25(2)43-28(5)17-18-47(62-43)23-34-20-33(61-47)16-15-27(4)42(26(3)13-12-14-32-24-55-45-40(49)29(6)19-35(46(51)58-34)48(32,45)52)59-39-22-37(54-10)44(31(8)57-39)60-38-21-36(53-9)41(50)30(7)56-38/h12-15,19,25-26,28,30-31,33-45,49-50,52H,11,16-18,20-24H2,1-10H3/b13-12+,27-15+,32-14+/t25-,26-,28-,30-,31-,33+,34-,35-,36-,37-,38-,39-,40+,41-,42-,43+,44-,45+,47+,48-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50436200
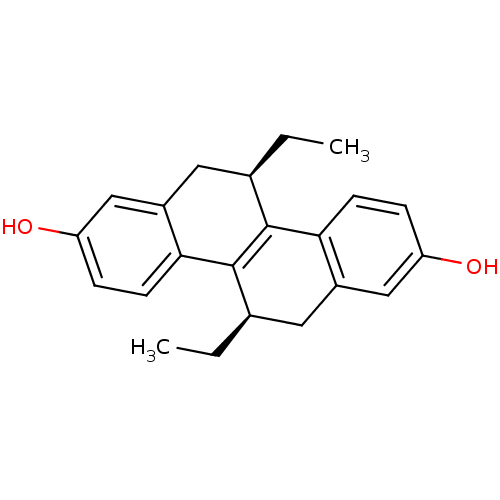
(CHEMBL282489)Show SMILES CC[C@@H]1Cc2cc(O)ccc2C2=C1c1ccc(O)cc1C[C@H]2CC |r,c:12| Show InChI InChI=1S/C22H24O2/c1-3-13-9-15-11-17(23)6-8-20(15)22-14(4-2)10-16-12-18(24)5-7-19(16)21(13)22/h5-8,11-14,23-24H,3-4,9-10H2,1-2H3/t13-,14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50054245
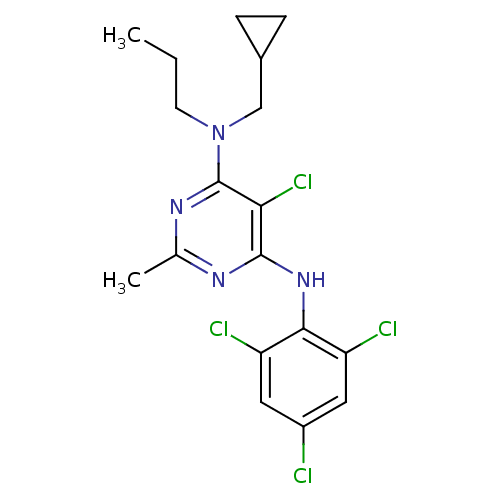
(5-Chloro-N-cyclopropylmethyl-2-methyl-N-propyl-N''...)Show SMILES CCCN(CC1CC1)c1nc(C)nc(Nc2c(Cl)cc(Cl)cc2Cl)c1Cl Show InChI InChI=1S/C18H20Cl4N4/c1-3-6-26(9-11-4-5-11)18-15(22)17(23-10(2)24-18)25-16-13(20)7-12(19)8-14(16)21/h7-8,11H,3-6,9H2,1-2H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM22876
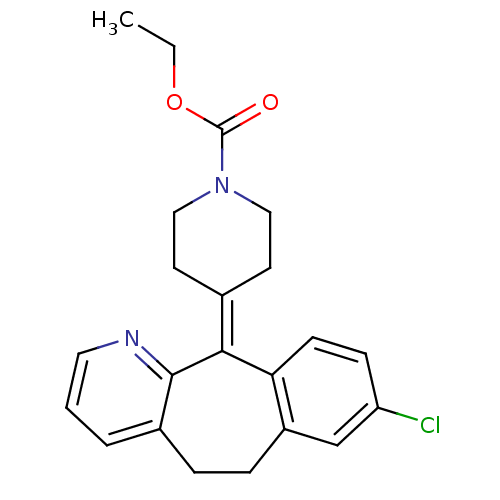
(CHEMBL998 | Claritin | Loratadine | Sch 29851 | US...)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C22H23ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21282

(2-methyl-3-(naphthalen-1-ylcarbonyl)-1-propyl-1H-i...)Show InChI InChI=1S/C23H21NO/c1-3-15-24-16(2)22(20-12-6-7-14-21(20)24)23(25)19-13-8-10-17-9-4-5-11-18(17)19/h4-14H,3,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM31774
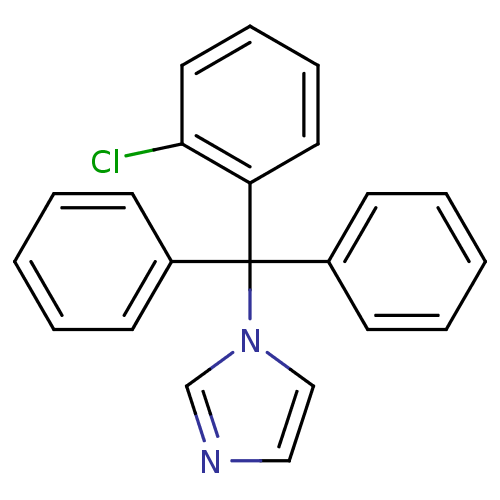
(CHEMBL104 | Canesten | Clotrimazole | Lotrimin | M...)Show InChI InChI=1S/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50191406

((Z)-6'-Bromo-1H,1'H-[2,3']biindolylidene-3,2'-dion...)Show SMILES Oc1[nH]c2cc(Br)ccc2c1-c1[nH]c2ccccc2c1N=O |(23.36,-3.09,;23.85,-1.62,;25.3,-1.15,;25.3,.39,;26.44,1.42,;26.12,2.93,;27.21,4.03,;24.65,3.4,;23.52,2.36,;23.85,.86,;22.94,-.38,;21.4,-.38,;20.49,-1.64,;19.02,-1.15,;17.67,-1.92,;16.34,-1.15,;16.34,.39,;17.67,1.16,;19.02,.39,;20.49,.88,;20.96,2.33,;19.93,3.48,)| Show InChI InChI=1S/C16H10BrN3O2/c17-8-5-6-9-12(7-8)19-16(21)13(9)15-14(20-22)10-3-1-2-4-11(10)18-15/h1-7,18-19,21H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50099491
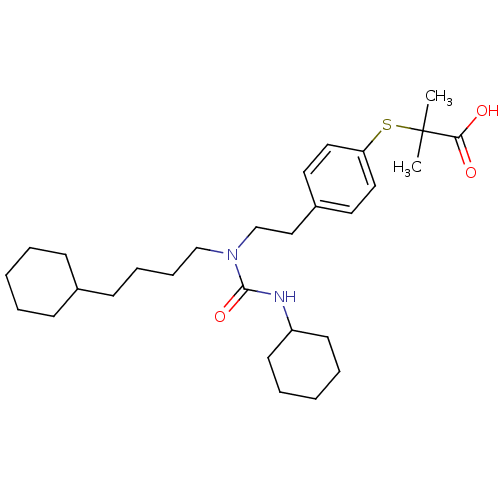
(2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...)Show SMILES CC(C)(Sc1ccc(CCN(CCCCC2CCCCC2)C(=O)NC2CCCCC2)cc1)C(O)=O Show InChI InChI=1S/C29H46N2O3S/c1-29(2,27(32)33)35-26-18-16-24(17-19-26)20-22-31(28(34)30-25-14-7-4-8-15-25)21-10-9-13-23-11-5-3-6-12-23/h16-19,23,25H,3-15,20-22H2,1-2H3,(H,30,34)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM41098

(FELODIPINE | MLS000069629 | SMR000058204 | cid_333...)Show SMILES CCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(Cl)c1Cl)C(=O)OC |c:5,t:8| Show InChI InChI=1S/C18H19Cl2NO4/c1-5-25-18(23)14-10(3)21-9(2)13(17(22)24-4)15(14)11-7-6-8-12(19)16(11)20/h6-8,13,15H,5H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM31770
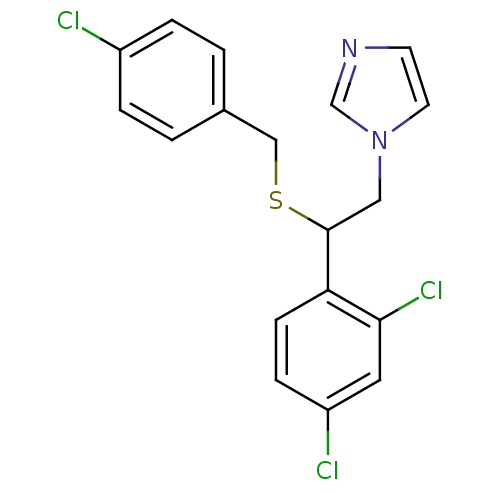
(Exelderm | Sulconazole Nitrate | cid_65495 | sulco...)Show InChI InChI=1S/C18H15Cl3N2S/c19-14-3-1-13(2-4-14)11-24-18(10-23-8-7-22-12-23)16-6-5-15(20)9-17(16)21/h1-9,12,18H,10-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50301375

(3,3',5,5'-tetraiodo-L-thyronine | 3,5,3',5'-tetrai...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2cc(I)c(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H11I4NO4/c16-8-4-7(5-9(17)13(8)21)24-14-10(18)1-6(2-11(14)19)3-12(20)15(22)23/h1-2,4-5,12,21H,3,20H2,(H,22,23)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50436204

(CHEMBL109037)Show InChI InChI=1S/C9H11NO5/c10-5(9(14)15)1-4-2-7(12)8(13)3-6(4)11/h2-3,5,11-13H,1,10H2,(H,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50318493
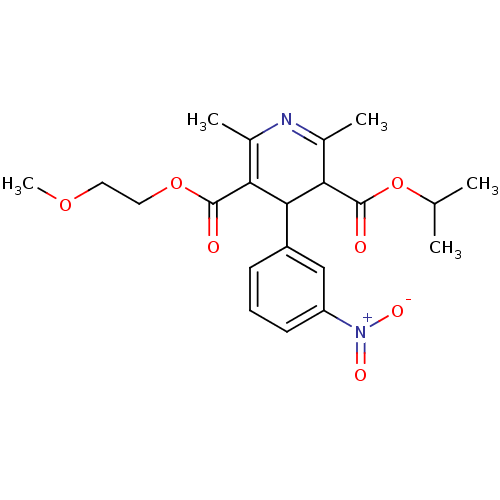
(3-isopropyl 5-(2-methoxyethyl) 2,6-dimethyl-4-(3-n...)Show SMILES COCCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC(C)C |c:7,t:10| Show InChI InChI=1S/C21H26N2O7/c1-12(2)30-21(25)18-14(4)22-13(3)17(20(24)29-10-9-28-5)19(18)15-7-6-8-16(11-15)23(26)27/h6-8,11-12,18-19H,9-10H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50436202

(CHEMBL1256761 | Palmitoyl-Dl-Carnitine Chloride)Show SMILES CCCCCCCCCCCCCCCC(=O)OC(CC(O)=O)C[N+](C)(C)C Show InChI InChI=1S/C23H45NO4/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-23(27)28-21(19-22(25)26)20-24(2,3)4/h21H,5-20H2,1-4H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21725
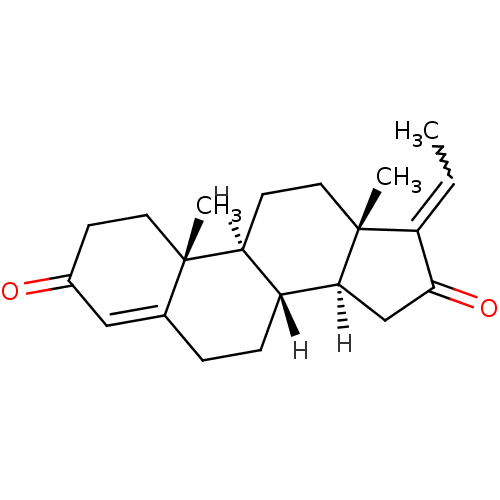
((1S,2R,10R,11S,14Z,15S)-14-ethylidene-2,15-dimethy...)Show SMILES [H][C@@]12CC(=O)C(=CC)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |w:6.6,t:20| Show InChI InChI=1S/C21H28O2/c1-4-16-19(23)12-18-15-6-5-13-11-14(22)7-9-20(13,2)17(15)8-10-21(16,18)3/h4,11,15,17-18H,5-10,12H2,1-3H3/t15-,17+,18+,20+,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at FXR (unknown origin) by coactivator assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM50436201

(FLUTRIMAZOLE | UR-4056)Show InChI InChI=1S/C22H16F2N2/c23-19-12-10-18(11-13-19)22(26-15-14-25-16-26,17-6-2-1-3-7-17)20-8-4-5-9-21(20)24/h1-16H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human GTS-tagged FXR after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at PXR (unknown origin) expressed in human HepG2 cells assessed as induction of CYP3A4 transactivation after 16 hrs by luciferase re... |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50436201

(FLUTRIMAZOLE | UR-4056)Show InChI InChI=1S/C22H16F2N2/c23-19-12-10-18(11-13-19)22(26-15-14-25-16-26,17-6-2-1-3-7-17)20-8-4-5-9-21(20)24/h1-16H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at PXR (unknown origin) expressed in human HepG2 cells assessed as induction of CYP3A4 transactivation after 16 hrs by luciferase re... |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50318493

(3-isopropyl 5-(2-methoxyethyl) 2,6-dimethyl-4-(3-n...)Show SMILES COCCOC(=O)C1=C(C)N=C(C)C(C1c1cccc(c1)[N+]([O-])=O)C(=O)OC(C)C |c:7,t:10| Show InChI InChI=1S/C21H26N2O7/c1-12(2)30-21(25)18-14(4)22-13(3)17(20(24)29-10-9-28-5)19(18)15-7-6-8-16(11-15)23(26)27/h6-8,11-12,18-19H,9-10H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 390 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at PXR (unknown origin) expressed in human HepG2 cells assessed as induction of CYP3A4 transactivation after 16 hrs by luciferase re... |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at PXR (unknown origin) expressed in human HepG2 cells assessed as induction of CYP3A4 transactivation after 16 hrs by luciferase re... |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM22876

(CHEMBL998 | Claritin | Loratadine | Sch 29851 | US...)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C22H23ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at PXR (unknown origin) expressed in human HepG2 cells assessed as induction of CYP3A4 transactivation after 16 hrs by luciferase re... |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM21282

(2-methyl-3-(naphthalen-1-ylcarbonyl)-1-propyl-1H-i...)Show InChI InChI=1S/C23H21NO/c1-3-15-24-16(2)22(20-12-6-7-14-21(20)24)23(25)19-13-8-10-17-9-4-5-11-18(17)19/h4-14H,3,15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at PXR (unknown origin) expressed in human HepG2 cells assessed as induction of CYP3A4 transactivation after 16 hrs by luciferase re... |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50054245

(5-Chloro-N-cyclopropylmethyl-2-methyl-N-propyl-N''...)Show SMILES CCCN(CC1CC1)c1nc(C)nc(Nc2c(Cl)cc(Cl)cc2Cl)c1Cl Show InChI InChI=1S/C18H20Cl4N4/c1-3-6-26(9-11-4-5-11)18-15(22)17(23-10(2)24-18)25-16-13(20)7-12(19)8-14(16)21/h7-8,11H,3-6,9H2,1-2H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.95E+3 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at PXR (unknown origin) expressed in human HepG2 cells assessed as induction of CYP3A4 transactivation after 16 hrs by luciferase re... |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 1 group I member 2
(Homo sapiens (Human)) | BDBM50436200

(CHEMBL282489)Show SMILES CC[C@@H]1Cc2cc(O)ccc2C2=C1c1ccc(O)cc1C[C@H]2CC |r,c:12| Show InChI InChI=1S/C22H24O2/c1-3-13-9-15-11-17(23)6-8-20(15)22-14(4-2)10-16-12-18(24)5-7-19(16)21(13)22/h5-8,11-14,23-24H,3-4,9-10H2,1-2H3/t13-,14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at PXR (unknown origin) expressed in human HepG2 cells assessed as induction of CYP3A4 transactivation after 16 hrs by luciferase re... |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724

(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 753 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to human GTS-tagged FXR LBD using fluorescein-tagged SRC2-2 after 30 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724

(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 373 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to human GTS-tagged FXR LBD using fluorescein-tagged SRC2-2 after 20 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724

(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Agonist activity at human FXR assessed as recruitment of SRC1 peptide by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bile acid receptor
(Homo sapiens (Human)) | BDBM21724

(3-[(E)-2-(2-chloro-4-{[3-(2,6-dichlorophenyl)-5-(1...)Show SMILES CC(C)c1onc(c1COc1ccc(\C=C\c2cccc(c2)C(O)=O)c(Cl)c1)-c1c(Cl)cccc1Cl |(-5,9.13,;-4.28,7.76,;-2.74,7.7,;-5.11,6.46,;-6.65,6.36,;-7.03,4.87,;-5.73,4.05,;-4.54,5.03,;-3.21,4.26,;-1.87,5.03,;-.54,4.26,;.79,5.03,;2.13,4.26,;2.13,2.72,;3.46,1.95,;4.79,2.72,;6.13,1.95,;6.13,.41,;7.46,-.36,;8.79,.41,;8.79,1.95,;7.46,2.72,;10.13,2.72,;11.46,1.95,;10.13,4.26,;.79,1.95,;.79,.41,;-.54,2.72,;-5.63,2.51,;-4.28,1.78,;-2.97,2.59,;-4.23,.24,;-5.54,-.57,;-6.9,.16,;-6.94,1.7,;-8.3,2.43,)| Show InChI InChI=1S/C28H22Cl3NO4/c1-16(2)27-21(26(32-36-27)25-22(29)7-4-8-23(25)30)15-35-20-12-11-18(24(31)14-20)10-9-17-5-3-6-19(13-17)28(33)34/h3-14,16H,15H2,1-2H3,(H,33,34)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 317 | n/a | n/a | n/a | n/a |
City of Hope National Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity to human GTS-tagged FXR LBD using fluorescein-tagged SRC2-2 after 15 mins by TR-FRET assay |
Bioorg Med Chem 21: 4266-78 (2013)
Article DOI: 10.1016/j.bmc.2013.04.069
BindingDB Entry DOI: 10.7270/Q2J67J9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data