 Found 65 hits Enz. Inhib. hit(s) with all data for entry = 50044165
Found 65 hits Enz. Inhib. hit(s) with all data for entry = 50044165 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007199
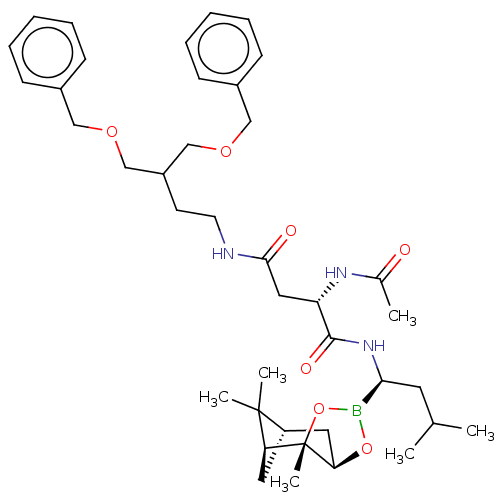
(CHEMBL3237863)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C40H58BN3O7/c1-27(2)19-36(41-50-35-21-32-20-34(39(32,4)5)40(35,6)51-41)44-38(47)33(43-28(3)45)22-37(46)42-18-17-31(25-48-23-29-13-9-7-10-14-29)26-49-24-30-15-11-8-12-16-30/h7-16,27,31-36H,17-26H2,1-6H3,(H,42,46)(H,43,45)(H,44,47)/t32-,33-,34-,35+,36-,40-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007198

(CHEMBL3237862)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C39H56BN3O7/c1-26(2)17-35(40-49-34-19-31-18-33(38(31,4)5)39(34,6)50-40)43-37(46)32(42-27(3)44)20-36(45)41-21-30(24-47-22-28-13-9-7-10-14-28)25-48-23-29-15-11-8-12-16-29/h7-16,26,30-35H,17-25H2,1-6H3,(H,41,45)(H,42,44)(H,43,46)/t31-,32-,33-,34+,35-,39-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007206
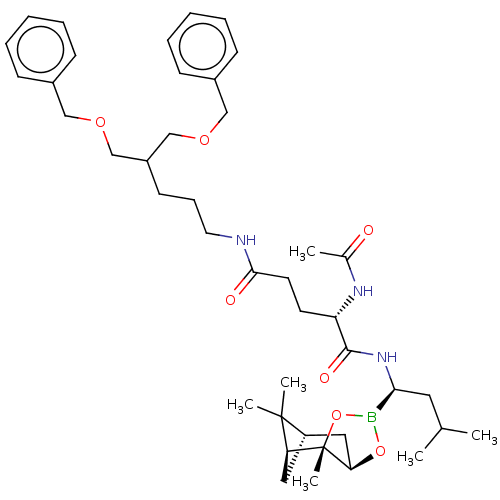
(CHEMBL3237867)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CCC(=O)NCCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C42H62BN3O7/c1-29(2)22-38(43-52-37-24-34-23-36(41(34,4)5)42(37,6)53-43)46-40(49)35(45-30(3)47)19-20-39(48)44-21-13-18-33(27-50-25-31-14-9-7-10-15-31)28-51-26-32-16-11-8-12-17-32/h7-12,14-17,29,33-38H,13,18-28H2,1-6H3,(H,44,48)(H,45,47)(H,46,49)/t34-,35-,36-,37+,38-,42-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007196
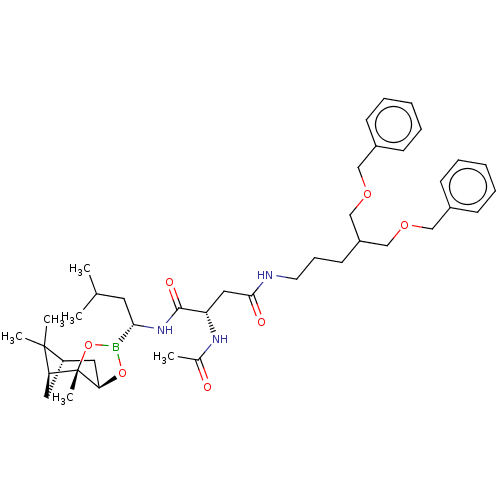
(CHEMBL3237864)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C41H60BN3O7/c1-28(2)20-37(42-51-36-22-33-21-35(40(33,4)5)41(36,6)52-42)45-39(48)34(44-29(3)46)23-38(47)43-19-13-18-32(26-49-24-30-14-9-7-10-15-30)27-50-25-31-16-11-8-12-17-31/h7-12,14-17,28,32-37H,13,18-27H2,1-6H3,(H,43,47)(H,44,46)(H,45,48)/t33-,34-,35-,36+,37-,41-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007200
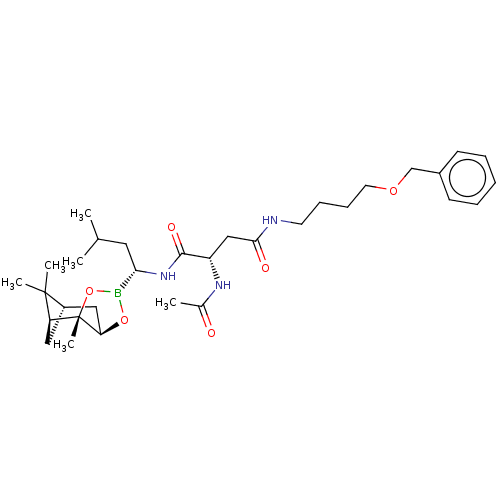
(CHEMBL3237873)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCCCOCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C32H50BN3O6/c1-21(2)16-28(33-41-27-18-24-17-26(31(24,4)5)32(27,6)42-33)36-30(39)25(35-22(3)37)19-29(38)34-14-10-11-15-40-20-23-12-8-7-9-13-23/h7-9,12-13,21,24-28H,10-11,14-20H2,1-6H3,(H,34,38)(H,35,37)(H,36,39)/t24-,25-,26-,27+,28-,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007193
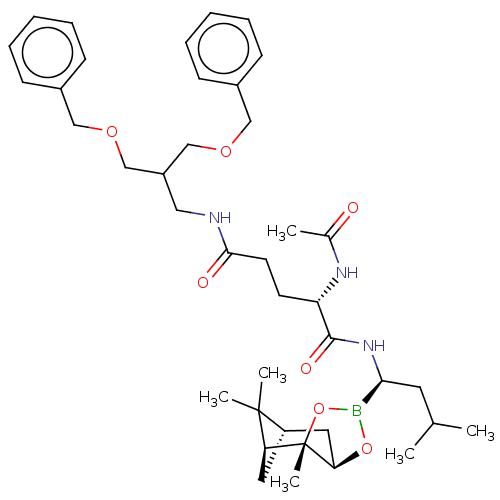
(CHEMBL3237865)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CCC(=O)NCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C40H58BN3O7/c1-27(2)19-36(41-50-35-21-32-20-34(39(32,4)5)40(35,6)51-41)44-38(47)33(43-28(3)45)17-18-37(46)42-22-31(25-48-23-29-13-9-7-10-14-29)26-49-24-30-15-11-8-12-16-30/h7-16,27,31-36H,17-26H2,1-6H3,(H,42,46)(H,43,45)(H,44,47)/t32-,33-,34-,35+,36-,40-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007207
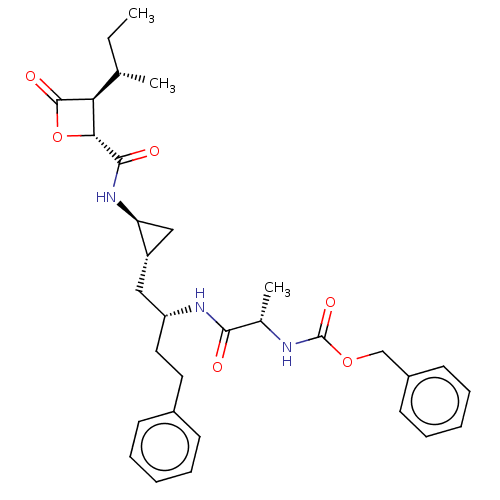
(CHEMBL3237860)Show SMILES [H][C@]1([C@@H](C)CC)[C@@H](OC1=O)C(=O)N[C@H]1C[C@@H]1C[C@@H](CCc1ccccc1)NC(=O)[C@H](C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C32H41N3O6/c1-4-20(2)27-28(41-31(27)38)30(37)35-26-18-24(26)17-25(16-15-22-11-7-5-8-12-22)34-29(36)21(3)33-32(39)40-19-23-13-9-6-10-14-23/h5-14,20-21,24-28H,4,15-19H2,1-3H3,(H,33,39)(H,34,36)(H,35,37)/t20-,21-,24-,25+,26-,27-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007195
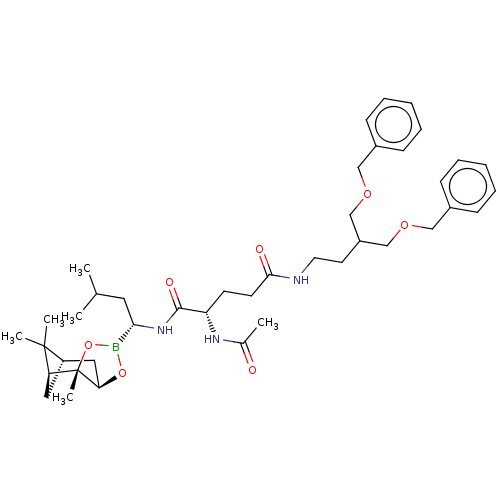
(CHEMBL3237866)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CCC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C41H60BN3O7/c1-28(2)21-37(42-51-36-23-33-22-35(40(33,4)5)41(36,6)52-42)45-39(48)34(44-29(3)46)17-18-38(47)43-20-19-32(26-49-24-30-13-9-7-10-14-30)27-50-25-31-15-11-8-12-16-31/h7-16,28,32-37H,17-27H2,1-6H3,(H,43,47)(H,44,46)(H,45,48)/t33-,34-,35-,36+,37-,41-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007202
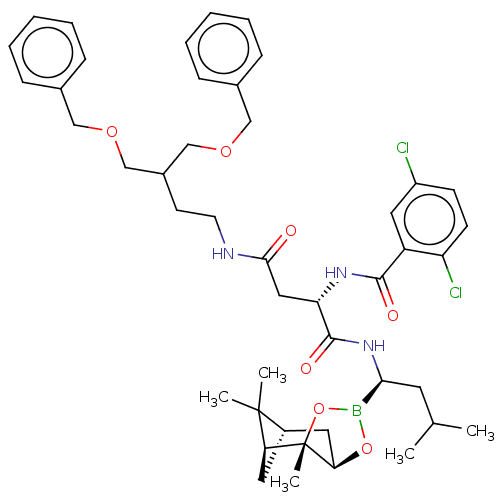
(CHEMBL3237868)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(=O)c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C45H58BCl2N3O7/c1-29(2)20-40(46-57-39-22-33-21-38(44(33,3)4)45(39,5)58-46)51-43(54)37(50-42(53)35-23-34(47)16-17-36(35)48)24-41(52)49-19-18-32(27-55-25-30-12-8-6-9-13-30)28-56-26-31-14-10-7-11-15-31/h6-17,23,29,32-33,37-40H,18-22,24-28H2,1-5H3,(H,49,52)(H,50,53)(H,51,54)/t33-,37-,38-,39+,40-,45-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007201

(CHEMBL3237870)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(=O)c1cnccn1 |r| Show InChI InChI=1S/C43H58BN5O7/c1-29(2)20-38(44-55-37-22-33-21-36(42(33,3)4)43(37,5)56-44)49-40(51)34(48-41(52)35-24-45-18-19-46-35)23-39(50)47-17-16-32(27-53-25-30-12-8-6-9-13-30)28-54-26-31-14-10-7-11-15-31/h6-15,18-19,24,29,32-34,36-38H,16-17,20-23,25-28H2,1-5H3,(H,47,50)(H,48,52)(H,49,51)/t33-,34-,36-,37+,38-,43-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007205
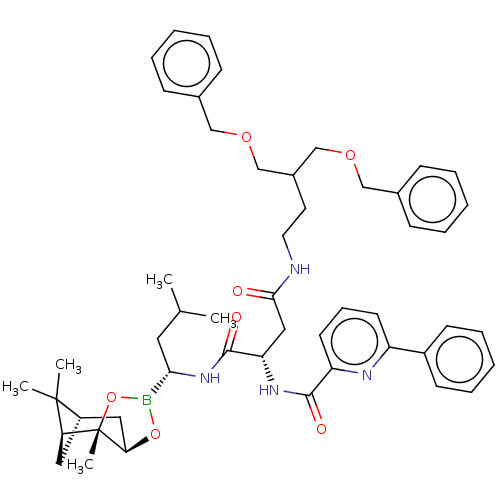
(CHEMBL3237871)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(=O)c1cccc(n1)-c1ccccc1 |r| Show InChI InChI=1S/C50H63BN4O7/c1-34(2)26-45(51-61-44-28-39-27-43(49(39,3)4)50(44,5)62-51)55-48(58)42(54-47(57)41-23-15-22-40(53-41)38-20-13-8-14-21-38)29-46(56)52-25-24-37(32-59-30-35-16-9-6-10-17-35)33-60-31-36-18-11-7-12-19-36/h6-23,34,37,39,42-45H,24-33H2,1-5H3,(H,52,56)(H,54,57)(H,55,58)/t39-,42-,43-,44+,45-,50-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007209
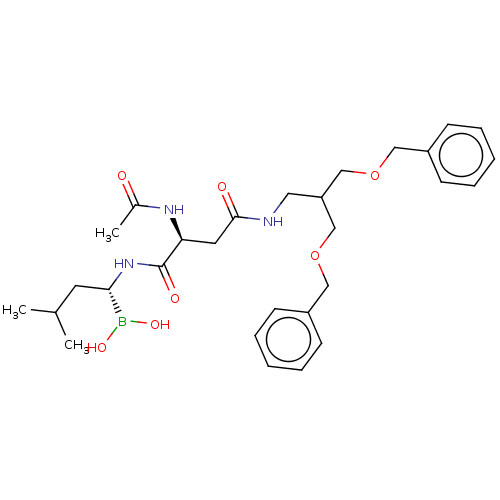
(CHEMBL3237874)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(=O)NCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O)B(O)O |r| Show InChI InChI=1S/C29H42BN3O7/c1-21(2)14-27(30(37)38)33-29(36)26(32-22(3)34)15-28(35)31-16-25(19-39-17-23-10-6-4-7-11-23)20-40-18-24-12-8-5-9-13-24/h4-13,21,25-27,37-38H,14-20H2,1-3H3,(H,31,35)(H,32,34)(H,33,36)/t26-,27-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007197
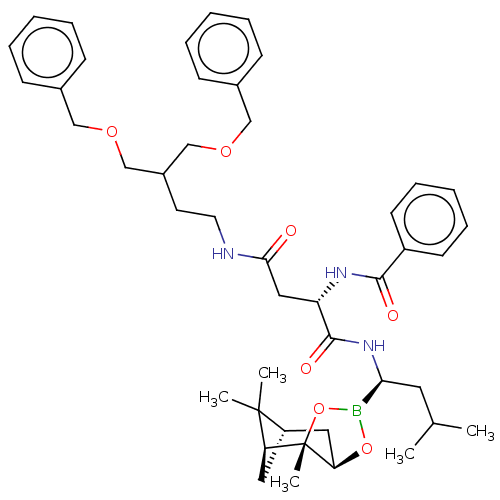
(CHEMBL3237869)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(=O)c1ccccc1 |r| Show InChI InChI=1S/C45H60BN3O7/c1-31(2)23-40(46-55-39-25-36-24-38(44(36,3)4)45(39,5)56-46)49-43(52)37(48-42(51)35-19-13-8-14-20-35)26-41(50)47-22-21-34(29-53-27-32-15-9-6-10-16-32)30-54-28-33-17-11-7-12-18-33/h6-20,31,34,36-40H,21-30H2,1-5H3,(H,47,50)(H,48,51)(H,49,52)/t36-,37-,38-,39+,40-,45-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007208
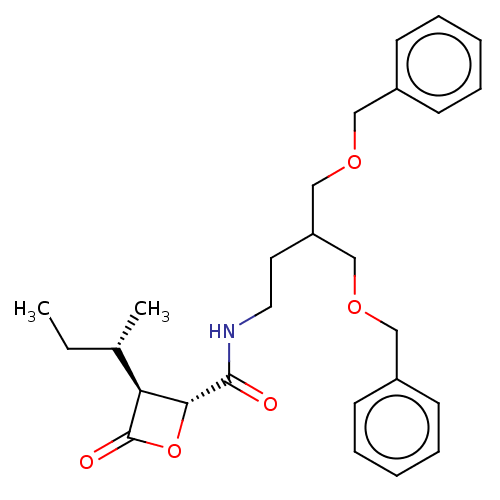
(CHEMBL3237861)Show SMILES [H][C@]1([C@@H](C)CC)[C@@H](OC1=O)C(=O)NCCC(COCc1ccccc1)COCc1ccccc1 |r| Show InChI InChI=1S/C27H35NO5/c1-3-20(2)24-25(33-27(24)30)26(29)28-15-14-23(18-31-16-21-10-6-4-7-11-21)19-32-17-22-12-8-5-9-13-22/h4-13,20,23-25H,3,14-19H2,1-2H3,(H,28,29)/t20-,24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50007199

(CHEMBL3237863)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C40H58BN3O7/c1-27(2)19-36(41-50-35-21-32-20-34(39(32,4)5)40(35,6)51-41)44-38(47)33(43-28(3)45)22-37(46)42-18-17-31(25-48-23-29-13-9-7-10-14-29)26-49-24-30-15-11-8-12-16-30/h7-16,27,31-36H,17-26H2,1-6H3,(H,42,46)(H,43,45)(H,44,47)/t32-,33-,34-,35+,36-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of beta5i subunit of immunoproteasome (unknown origin) by active-site ELISA |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007206

(CHEMBL3237867)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CCC(=O)NCCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C42H62BN3O7/c1-29(2)22-38(43-52-37-24-34-23-36(41(34,4)5)42(37,6)53-43)46-40(49)35(45-30(3)47)19-20-39(48)44-21-13-18-33(27-50-25-31-14-9-7-10-15-31)28-51-26-32-16-11-8-12-17-32/h7-12,14-17,29,33-38H,13,18-28H2,1-6H3,(H,44,48)(H,45,47)(H,46,49)/t34-,35-,36-,37+,38-,42-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007204

(CHEMBL3237872)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NC)NC(C)=O |r| Show InChI InChI=1S/C22H38BN3O5/c1-12(2)8-18(26-20(29)15(25-13(3)27)11-19(28)24-7)23-30-17-10-14-9-16(21(14,4)5)22(17,6)31-23/h12,14-18H,8-11H2,1-7H3,(H,24,28)(H,25,27)(H,26,29)/t14-,15-,16-,17+,18-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50007200

(CHEMBL3237873)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCCCOCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C32H50BN3O6/c1-21(2)16-28(33-41-27-18-24-17-26(31(24,4)5)32(27,6)42-33)36-30(39)25(35-22(3)37)19-29(38)34-14-10-11-15-40-20-23-12-8-7-9-13-23/h7-9,12-13,21,24-28H,10-11,14-20H2,1-6H3,(H,34,38)(H,35,37)(H,36,39)/t24-,25-,26-,27+,28-,32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of beta5i subunit of immunoproteasome (unknown origin) by active-site ELISA |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
A5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436
(Homo sapiens (Human)) | BDBM50007193

(CHEMBL3237865)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CCC(=O)NCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C40H58BN3O7/c1-27(2)19-36(41-50-35-21-32-20-34(39(32,4)5)40(35,6)51-41)44-38(47)33(43-28(3)45)17-18-37(46)42-22-31(25-48-23-29-13-9-7-10-14-29)26-49-24-30-15-11-8-12-16-30/h7-16,27,31-36H,17-26H2,1-6H3,(H,42,46)(H,43,45)(H,44,47)/t32-,33-,34-,35+,36-,40-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007195

(CHEMBL3237866)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CCC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C41H60BN3O7/c1-28(2)21-37(42-51-36-23-33-22-35(40(33,4)5)41(36,6)52-42)45-39(48)34(44-29(3)46)17-18-38(47)43-20-19-32(26-49-24-30-13-9-7-10-14-30)27-50-25-31-15-11-8-12-16-31/h7-16,28,32-37H,17-27H2,1-6H3,(H,43,47)(H,44,46)(H,45,48)/t33-,34-,35-,36+,37-,41-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of beta5i subunit of immunoproteasome (unknown origin) by active-site ELISA |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007196

(CHEMBL3237864)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C41H60BN3O7/c1-28(2)20-37(42-51-36-22-33-21-35(40(33,4)5)41(36,6)52-42)45-39(48)34(44-29(3)46)23-38(47)43-19-13-18-32(26-49-24-30-14-9-7-10-15-30)27-50-25-31-16-11-8-12-17-31/h7-12,14-17,28,32-37H,13,18-27H2,1-6H3,(H,43,47)(H,44,46)(H,45,48)/t33-,34-,35-,36+,37-,41-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007197

(CHEMBL3237869)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(=O)c1ccccc1 |r| Show InChI InChI=1S/C45H60BN3O7/c1-31(2)23-40(46-55-39-25-36-24-38(44(36,3)4)45(39,5)56-46)49-43(52)37(48-42(51)35-19-13-8-14-20-35)26-41(50)47-22-21-34(29-53-27-32-15-9-6-10-16-32)30-54-28-33-17-11-7-12-18-33/h6-20,31,34,36-40H,21-30H2,1-5H3,(H,47,50)(H,48,51)(H,49,52)/t36-,37-,38-,39+,40-,45-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007198

(CHEMBL3237862)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C39H56BN3O7/c1-26(2)17-35(40-49-34-19-31-18-33(38(31,4)5)39(34,6)50-40)43-37(46)32(42-27(3)44)20-36(45)41-21-30(24-47-22-28-13-9-7-10-14-28)25-48-23-29-15-11-8-12-16-29/h7-16,26,30-35H,17-25H2,1-6H3,(H,41,45)(H,42,44)(H,43,46)/t31-,32-,33-,34+,35-,39-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of beta5 subunit of immunoproteasome (unknown origin) by active-site ELISA |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50007199

(CHEMBL3237863)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C40H58BN3O7/c1-27(2)19-36(41-50-35-21-32-20-34(39(32,4)5)40(35,6)51-41)44-38(47)33(43-28(3)45)22-37(46)42-18-17-31(25-48-23-29-13-9-7-10-14-29)26-49-24-30-15-11-8-12-16-30/h7-16,27,31-36H,17-26H2,1-6H3,(H,42,46)(H,43,45)(H,44,47)/t32-,33-,34-,35+,36-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of beta5 subunit of immunoproteasome (unknown origin) by active-site ELISA |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007199

(CHEMBL3237863)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C40H58BN3O7/c1-27(2)19-36(41-50-35-21-32-20-34(39(32,4)5)40(35,6)51-41)44-38(47)33(43-28(3)45)22-37(46)42-18-17-31(25-48-23-29-13-9-7-10-14-29)26-49-24-30-15-11-8-12-16-30/h7-16,27,31-36H,17-26H2,1-6H3,(H,42,46)(H,43,45)(H,44,47)/t32-,33-,34-,35+,36-,40-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007200

(CHEMBL3237873)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCCCOCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C32H50BN3O6/c1-21(2)16-28(33-41-27-18-24-17-26(31(24,4)5)32(27,6)42-33)36-30(39)25(35-22(3)37)19-29(38)34-14-10-11-15-40-20-23-12-8-7-9-13-23/h7-9,12-13,21,24-28H,10-11,14-20H2,1-6H3,(H,34,38)(H,35,37)(H,36,39)/t24-,25-,26-,27+,28-,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007201

(CHEMBL3237870)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(=O)c1cnccn1 |r| Show InChI InChI=1S/C43H58BN5O7/c1-29(2)20-38(44-55-37-22-33-21-36(42(33,3)4)43(37,5)56-44)49-40(51)34(48-41(52)35-24-45-18-19-46-35)23-39(50)47-17-16-32(27-53-25-30-12-8-6-9-13-30)28-54-26-31-14-10-7-11-15-31/h6-15,18-19,24,29,32-34,36-38H,16-17,20-23,25-28H2,1-5H3,(H,47,50)(H,48,52)(H,49,51)/t33-,34-,36-,37+,38-,43-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007202

(CHEMBL3237868)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(=O)c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C45H58BCl2N3O7/c1-29(2)20-40(46-57-39-22-33-21-38(44(33,3)4)45(39,5)58-46)51-43(54)37(50-42(53)35-23-34(47)16-17-36(35)48)24-41(52)49-19-18-32(27-55-25-30-12-8-6-9-13-30)28-56-26-31-14-10-7-11-15-31/h6-17,23,29,32-33,37-40H,18-22,24-28H2,1-5H3,(H,49,52)(H,50,53)(H,51,54)/t33-,37-,38-,39+,40-,45-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50007200

(CHEMBL3237873)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCCCOCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C32H50BN3O6/c1-21(2)16-28(33-41-27-18-24-17-26(31(24,4)5)32(27,6)42-33)36-30(39)25(35-22(3)37)19-29(38)34-14-10-11-15-40-20-23-12-8-7-9-13-23/h7-9,12-13,21,24-28H,10-11,14-20H2,1-6H3,(H,34,38)(H,35,37)(H,36,39)/t24-,25-,26-,27+,28-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of beta5 subunit of immunoproteasome (unknown origin) by active-site ELISA |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50007203
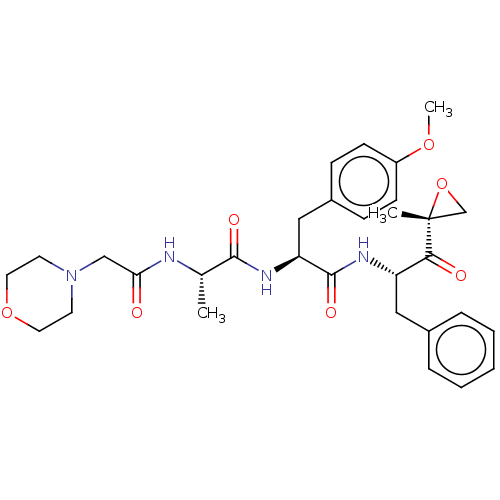
(CHEMBL3237875)Show SMILES COc1ccc(C[C@H](NC(=O)[C@H](C)NC(=O)CN2CCOCC2)C(=O)N[C@@H](Cc2ccccc2)C(=O)[C@@]2(C)CO2)cc1 |r| Show InChI InChI=1S/C31H40N4O7/c1-21(32-27(36)19-35-13-15-41-16-14-35)29(38)34-26(18-23-9-11-24(40-3)12-10-23)30(39)33-25(28(37)31(2)20-42-31)17-22-7-5-4-6-8-22/h4-12,21,25-26H,13-20H2,1-3H3,(H,32,36)(H,33,39)(H,34,38)/t21-,25-,26-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of beta5i subunit of immunoproteasome (unknown origin) by active-site ELISA |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007204

(CHEMBL3237872)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NC)NC(C)=O |r| Show InChI InChI=1S/C22H38BN3O5/c1-12(2)8-18(26-20(29)15(25-13(3)27)11-19(28)24-7)23-30-17-10-14-9-16(21(14,4)5)22(17,6)31-23/h12,14-18H,8-11H2,1-7H3,(H,24,28)(H,25,27)(H,26,29)/t14-,15-,16-,17+,18-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50432548

(LACTACYSTIN)Show SMILES CC(C)[C@H](O)[C@]1(NC(=O)[C@H](C)[C@@H]1O)C(=O)SC[C@H](NC(C)=O)C(O)=O |r| Show InChI InChI=1S/C15H24N2O7S/c1-6(2)10(19)15(11(20)7(3)12(21)17-15)14(24)25-5-9(13(22)23)16-8(4)18/h6-7,9-11,19-20H,5H2,1-4H3,(H,16,18)(H,17,21)(H,22,23)/t7-,9+,10+,11+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of beta5 subunit of immunoproteasome (unknown origin) by active-site ELISA |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007205

(CHEMBL3237871)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(=O)c1cccc(n1)-c1ccccc1 |r| Show InChI InChI=1S/C50H63BN4O7/c1-34(2)26-45(51-61-44-28-39-27-43(49(39,3)4)50(44,5)62-51)55-48(58)42(54-47(57)41-23-15-22-40(53-41)38-20-13-8-14-21-38)29-46(56)52-25-24-37(32-59-30-35-16-9-6-10-17-35)33-60-31-36-18-11-7-12-19-36/h6-23,34,37,39,42-45H,24-33H2,1-5H3,(H,52,56)(H,54,57)(H,55,58)/t39-,42-,43-,44+,45-,50-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of caspase-like activity of human 20S proteasome using Ac-nLPnLD-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50007203

(CHEMBL3237875)Show SMILES COc1ccc(C[C@H](NC(=O)[C@H](C)NC(=O)CN2CCOCC2)C(=O)N[C@@H](Cc2ccccc2)C(=O)[C@@]2(C)CO2)cc1 |r| Show InChI InChI=1S/C31H40N4O7/c1-21(32-27(36)19-35-13-15-41-16-14-35)29(38)34-26(18-23-9-11-24(40-3)12-10-23)30(39)33-25(28(37)31(2)20-42-31)17-22-7-5-4-6-8-22/h4-12,21,25-26H,13-20H2,1-3H3,(H,32,36)(H,33,39)(H,34,38)/t21-,25-,26-,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of beta5 subunit of immunoproteasome (unknown origin) by active-site ELISA |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007198

(CHEMBL3237862)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C39H56BN3O7/c1-26(2)17-35(40-49-34-19-31-18-33(38(31,4)5)39(34,6)50-40)43-37(46)32(42-27(3)44)20-36(45)41-21-30(24-47-22-28-13-9-7-10-14-28)25-48-23-29-15-11-8-12-16-29/h7-16,26,30-35H,17-25H2,1-6H3,(H,41,45)(H,42,44)(H,43,46)/t31-,32-,33-,34+,35-,39-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human 20S proteasome using Ac-RLR-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50430990
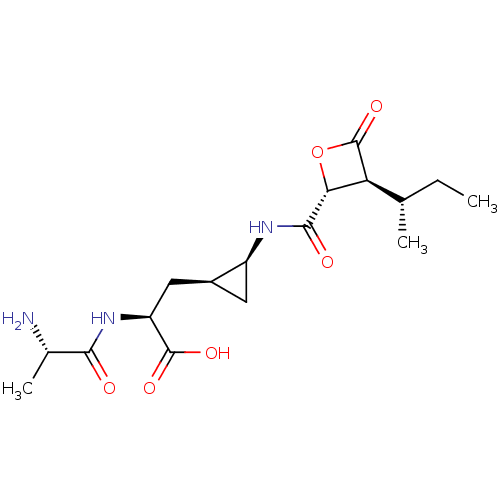
(BELACTOSIN A)Show SMILES CC[C@H](C)[C@H]1[C@@H](OC1=O)C(=O)N[C@H]1C[C@H]1C[C@H](NC(=O)[C@H](C)N)C(O)=O |r| Show InChI InChI=1S/C17H27N3O6/c1-4-7(2)12-13(26-17(12)25)15(22)19-10-5-9(10)6-11(16(23)24)20-14(21)8(3)18/h7-13H,4-6,18H2,1-3H3,(H,19,22)(H,20,21)(H,23,24)/t7-,8-,9-,10-,11-,12-,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of human 20S proteasome using Suc-LLVY-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human 20S proteasome using Ac-RLR-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007200

(CHEMBL3237873)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCCCOCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C32H50BN3O6/c1-21(2)16-28(33-41-27-18-24-17-26(31(24,4)5)32(27,6)42-33)36-30(39)25(35-22(3)37)19-29(38)34-14-10-11-15-40-20-23-12-8-7-9-13-23/h7-9,12-13,21,24-28H,10-11,14-20H2,1-6H3,(H,34,38)(H,35,37)(H,36,39)/t24-,25-,26-,27+,28-,32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human 20S proteasome using Ac-RLR-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007193

(CHEMBL3237865)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CCC(=O)NCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C40H58BN3O7/c1-27(2)19-36(41-50-35-21-32-20-34(39(32,4)5)40(35,6)51-41)44-38(47)33(43-28(3)45)17-18-37(46)42-22-31(25-48-23-29-13-9-7-10-14-29)26-49-24-30-15-11-8-12-16-30/h7-16,27,31-36H,17-26H2,1-6H3,(H,42,46)(H,43,45)(H,44,47)/t32-,33-,34-,35+,36-,40-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human 20S proteasome using Ac-RLR-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007195

(CHEMBL3237866)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CCC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C41H60BN3O7/c1-28(2)21-37(42-51-36-23-33-22-35(40(33,4)5)41(36,6)52-42)45-39(48)34(44-29(3)46)17-18-38(47)43-20-19-32(26-49-24-30-13-9-7-10-14-30)27-50-25-31-15-11-8-12-16-31/h7-16,28,32-37H,17-27H2,1-6H3,(H,43,47)(H,44,46)(H,45,48)/t33-,34-,35-,36+,37-,41-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human 20S proteasome using Ac-RLR-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007197

(CHEMBL3237869)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(=O)c1ccccc1 |r| Show InChI InChI=1S/C45H60BN3O7/c1-31(2)23-40(46-55-39-25-36-24-38(44(36,3)4)45(39,5)56-46)49-43(52)37(48-42(51)35-19-13-8-14-20-35)26-41(50)47-22-21-34(29-53-27-32-15-9-6-10-16-32)30-54-28-33-17-11-7-12-18-33/h6-20,31,34,36-40H,21-30H2,1-5H3,(H,47,50)(H,48,51)(H,49,52)/t36-,37-,38-,39+,40-,45-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human 20S proteasome using Ac-RLR-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007196

(CHEMBL3237864)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C41H60BN3O7/c1-28(2)20-37(42-51-36-22-33-21-35(40(33,4)5)41(36,6)52-42)45-39(48)34(44-29(3)46)23-38(47)43-19-13-18-32(26-49-24-30-14-9-7-10-15-30)27-50-25-31-16-11-8-12-17-31/h7-12,14-17,28,32-37H,13,18-27H2,1-6H3,(H,43,47)(H,44,46)(H,45,48)/t33-,34-,35-,36+,37-,41-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human 20S proteasome using Ac-RLR-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007199

(CHEMBL3237863)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)NCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C40H58BN3O7/c1-27(2)19-36(41-50-35-21-32-20-34(39(32,4)5)40(35,6)51-41)44-38(47)33(43-28(3)45)22-37(46)42-18-17-31(25-48-23-29-13-9-7-10-14-29)26-49-24-30-15-11-8-12-16-30/h7-16,27,31-36H,17-26H2,1-6H3,(H,42,46)(H,43,45)(H,44,47)/t32-,33-,34-,35+,36-,40-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human 20S proteasome using Ac-RLR-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-7
(Homo sapiens (Human)) | BDBM50007206

(CHEMBL3237867)Show SMILES [H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@H](CC(C)C)NC(=O)[C@H](CCC(=O)NCCCC(COCc1ccccc1)COCc1ccccc1)NC(C)=O |r| Show InChI InChI=1S/C42H62BN3O7/c1-29(2)22-38(43-52-37-24-34-23-36(41(34,4)5)42(37,6)53-43)46-40(49)35(45-30(3)47)19-20-39(48)44-21-13-18-33(27-50-25-31-14-9-7-10-15-31)28-51-26-32-16-11-8-12-17-32/h7-12,14-17,29,33-38H,13,18-28H2,1-6H3,(H,44,48)(H,45,47)(H,46,49)/t34-,35-,36-,37+,38-,42-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of trypsin-like activity of human 20S proteasome using Ac-RLR-AMC as substrate |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50432548

(LACTACYSTIN)Show SMILES CC(C)[C@H](O)[C@]1(NC(=O)[C@H](C)[C@@H]1O)C(=O)SC[C@H](NC(C)=O)C(O)=O |r| Show InChI InChI=1S/C15H24N2O7S/c1-6(2)10(19)15(11(20)7(3)12(21)17-15)14(24)25-5-9(13(22)23)16-8(4)18/h6-7,9-11,19-20H,5H2,1-4H3,(H,16,18)(H,17,21)(H,22,23)/t7-,9+,10+,11+,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of beta5i subunit of immunoproteasome (unknown origin) by active-site ELISA |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant cathepsin G (unknown origin) using Suc-Ala-Ala-Pro-Phe-AMC as substrate by spectrofluorometer analysis |
J Med Chem 57: 2726-35 (2014)
Article DOI: 10.1021/jm500045x
BindingDB Entry DOI: 10.7270/Q24M961D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data