

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442991 (CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of va... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM48009 (8-fluoranylindolo[2,1-b]quinazoline-6,12-dione | 8...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of va... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442987 (8-Bromotryptanthrin | CHEMBL72165) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of va... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442988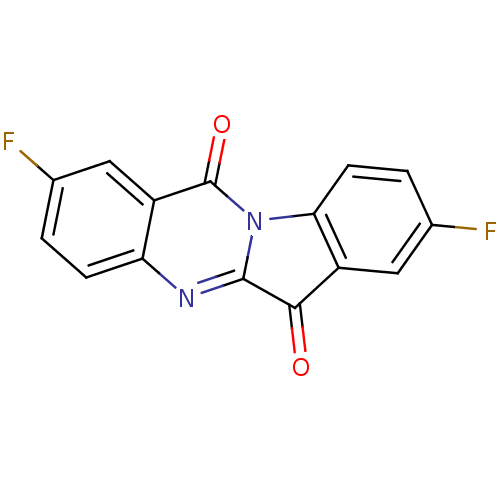 (CHEMBL3087009) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Mixed competitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence o... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50240612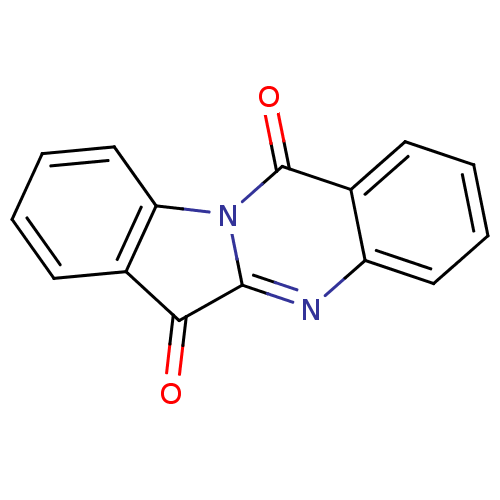 (CHEMBL306946 | GNF-PF-2691 | Indolo[2,1-b]quinazol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of va... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442989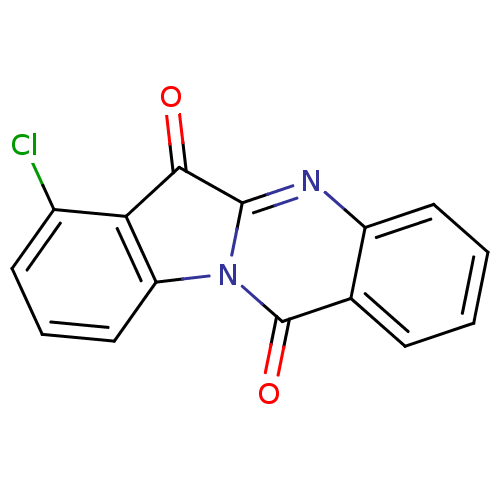 (CHEMBL3087010) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of va... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442994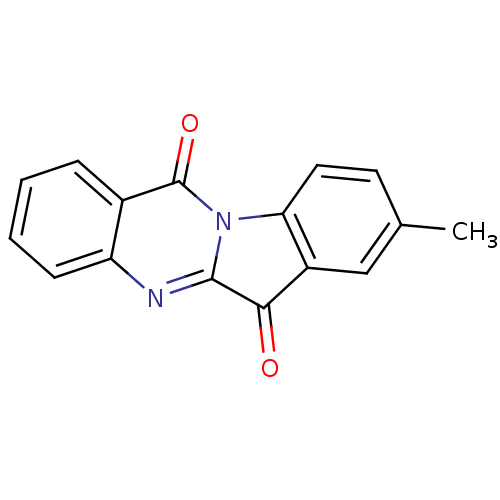 (CHEMBL312537 | US10669273, Compound 5b) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Non-competitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of ... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442990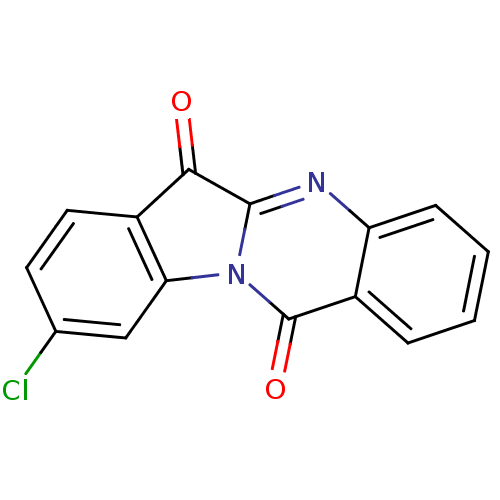 (CHEMBL1276265) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of va... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442995 (CHEMBL3086870) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Uncompetitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of va... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50241727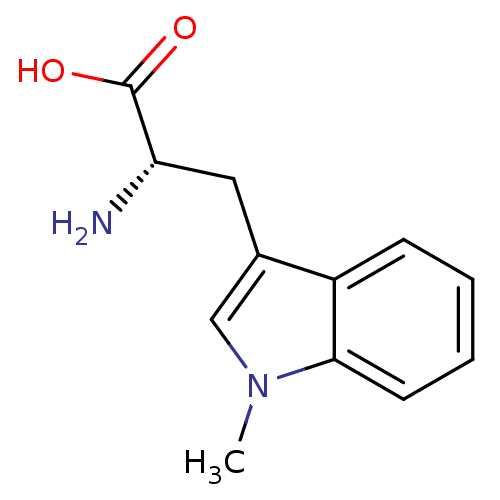 ((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynurenine formation using ... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50241727 ((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) in presence of vary... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50207089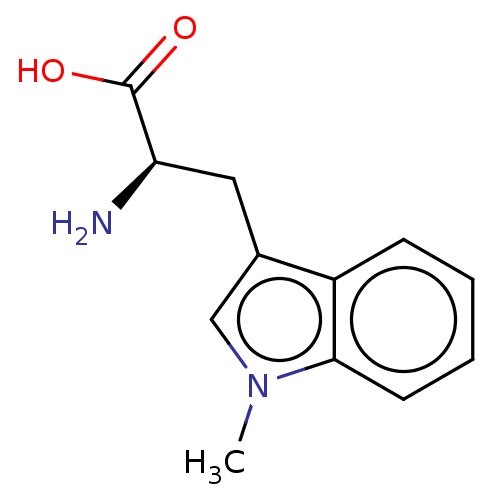 (D-1-Methyltryptophan | D-1MT | Indoximod) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynurenine formation using ... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442991 (CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM48009 (8-fluoranylindolo[2,1-b]quinazoline-6,12-dione | 8...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442991 (CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442987 (8-Bromotryptanthrin | CHEMBL72165) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50240612 (CHEMBL306946 | GNF-PF-2691 | Indolo[2,1-b]quinazol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM48009 (8-fluoranylindolo[2,1-b]quinazoline-6,12-dione | 8...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442988 (CHEMBL3087009) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442987 (8-Bromotryptanthrin | CHEMBL72165) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50240612 (CHEMBL306946 | GNF-PF-2691 | Indolo[2,1-b]quinazol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442990 (CHEMBL1276265) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442989 (CHEMBL3087010) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442988 (CHEMBL3087009) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442994 (CHEMBL312537 | US10669273, Compound 5b) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442994 (CHEMBL312537 | US10669273, Compound 5b) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442995 (CHEMBL3086870) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442989 (CHEMBL3087010) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442995 (CHEMBL3086870) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50241727 ((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442990 (CHEMBL1276265) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50241727 ((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50207089 (D-1-Methyltryptophan | D-1MT | Indoximod) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50207089 (D-1-Methyltryptophan | D-1MT | Indoximod) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||