

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50210778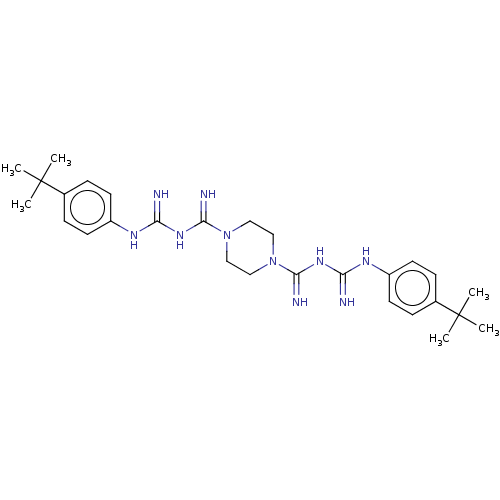 (CHEMBL3922793) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210771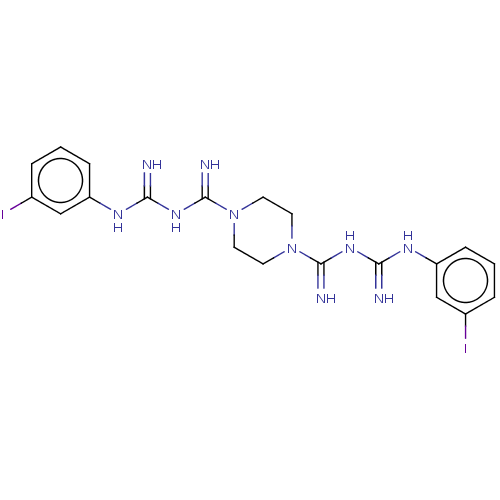 (CHEMBL3941312) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210781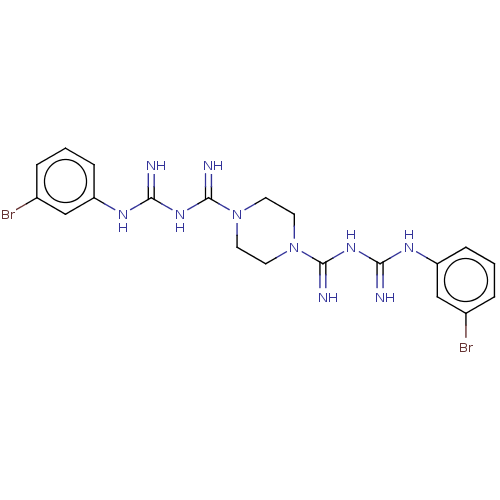 (CHEMBL3921743) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210782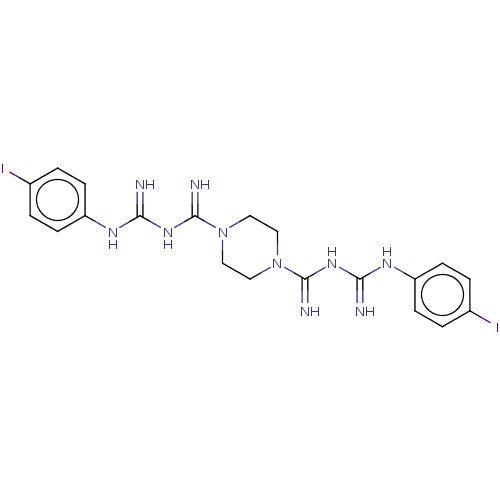 (CHEMBL3905115) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210773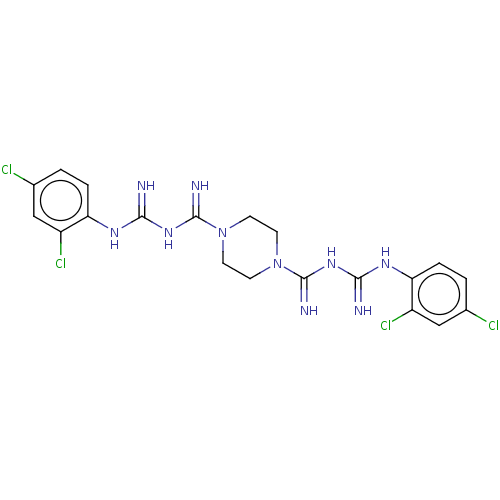 (CHEMBL3968731) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210779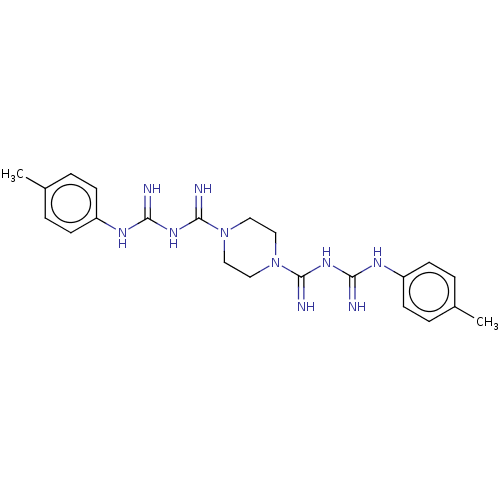 (CHEMBL3950513) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210772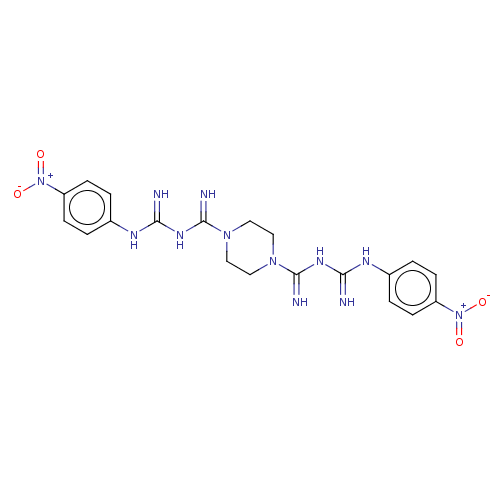 (CHEMBL3916022) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210170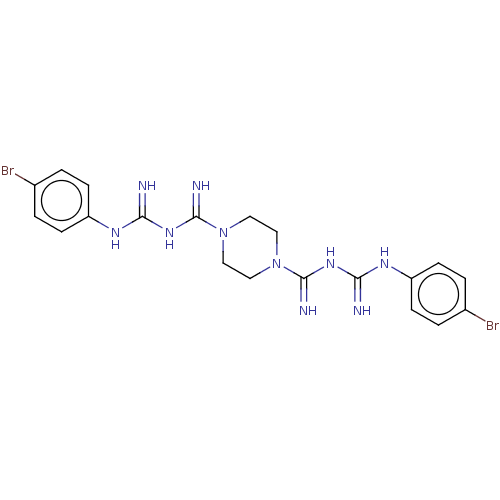 (CHEMBL3894580) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210775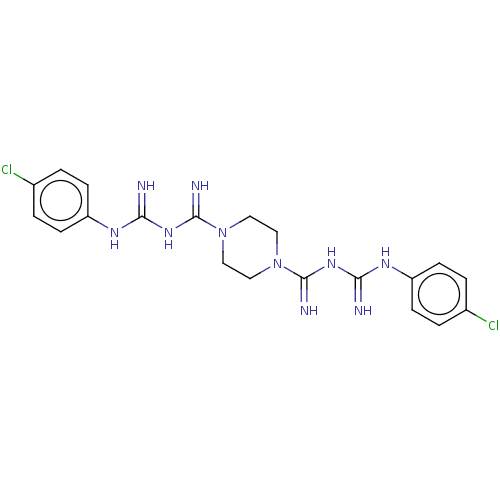 (Picloxydine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210787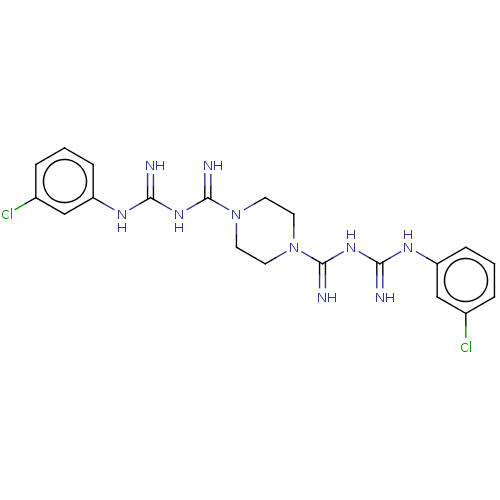 (CHEMBL3957684) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210769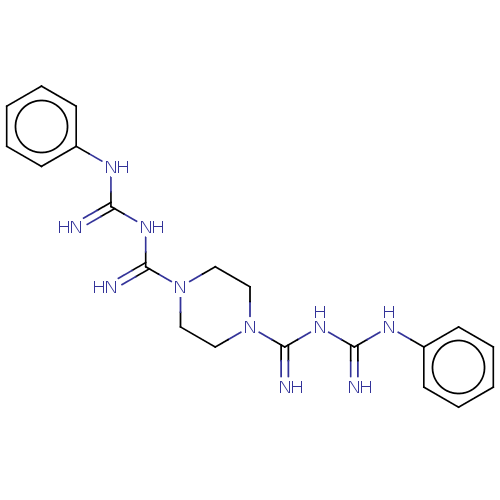 (CHEMBL3963893) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210780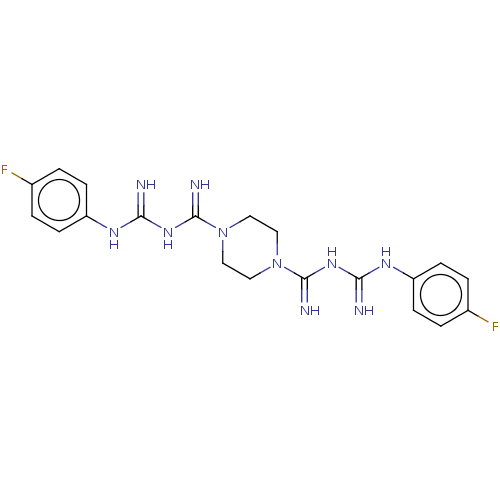 (CHEMBL3949066) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210770 (CHEMBL3897572) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210778 (CHEMBL3922793) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210784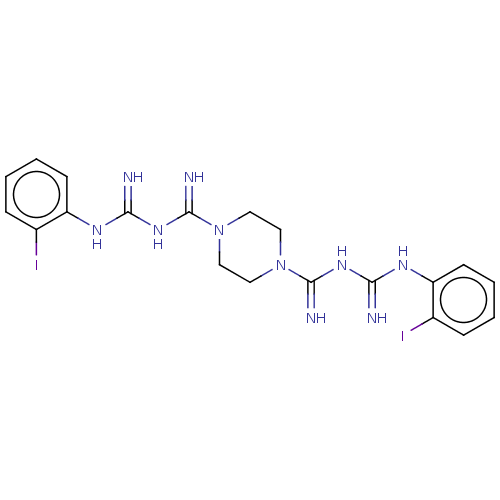 (CHEMBL3913329) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210786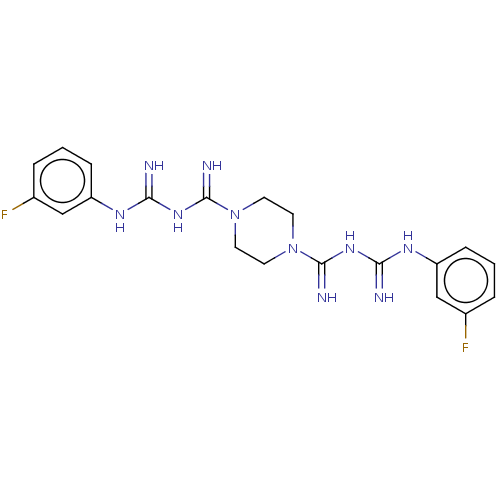 (CHEMBL3924020) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210783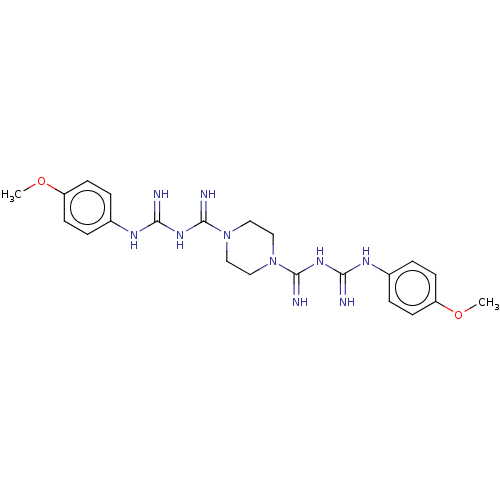 (CHEMBL3940753) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210171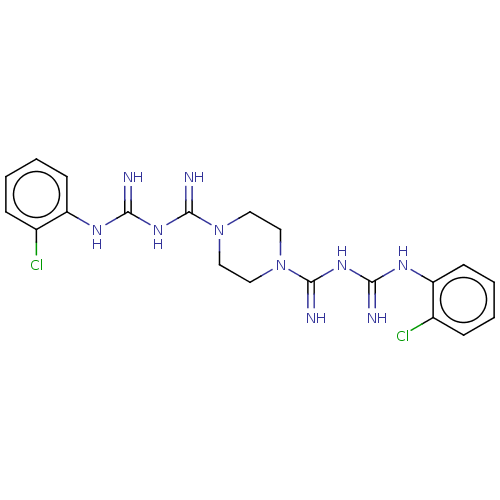 (CHEMBL3978195) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210784 (CHEMBL3913329) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210782 (CHEMBL3905115) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210771 (CHEMBL3941312) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210772 (CHEMBL3916022) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Non-competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincuba... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210785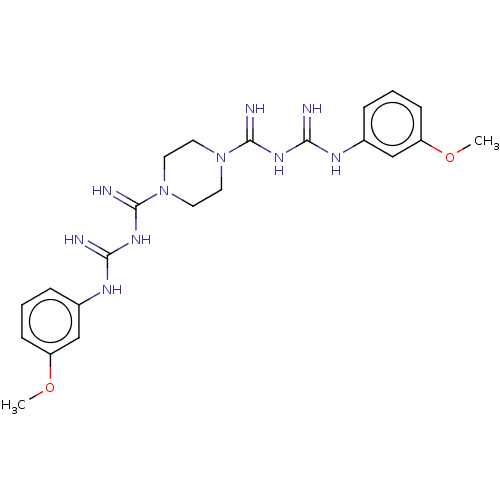 (CHEMBL3975982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210771 (CHEMBL3941312) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210776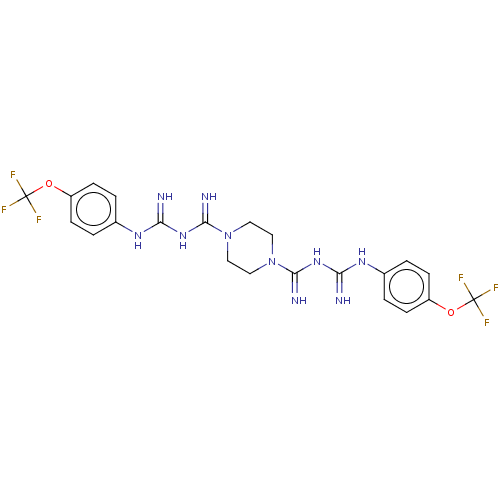 (CHEMBL3947927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210170 (CHEMBL3894580) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210170 (CHEMBL3894580) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210170 (CHEMBL3894580) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210770 (CHEMBL3897572) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210777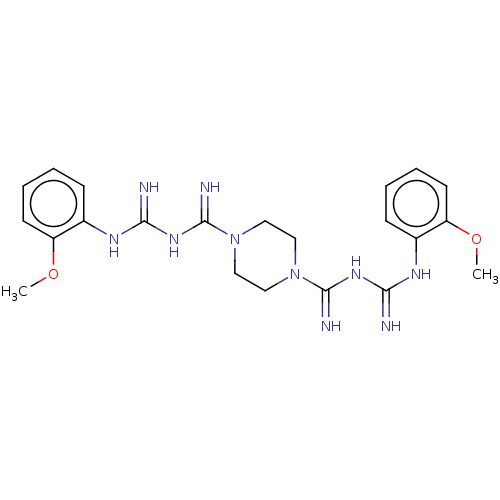 (CHEMBL3949522) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210778 (CHEMBL3922793) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210787 (CHEMBL3957684) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210782 (CHEMBL3905115) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210777 (CHEMBL3949522) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210171 (CHEMBL3978195) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210171 (CHEMBL3978195) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210781 (CHEMBL3921743) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210774 (CHEMBL3964220) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210773 (CHEMBL3968731) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210169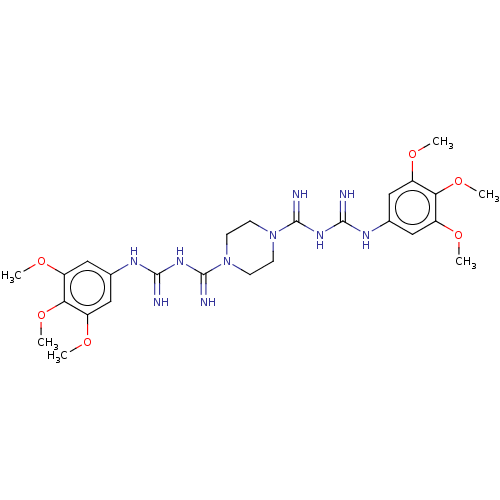 (CHEMBL3959216) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210780 (CHEMBL3949066) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210169 (CHEMBL3959216) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210779 (CHEMBL3950513) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210773 (CHEMBL3968731) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210775 (Picloxydine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 9.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210781 (CHEMBL3921743) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Non-competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50210785 (CHEMBL3975982) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of Electrophorus electricus acetylcholinesterase in presence of varying acetylthiocholine iodide substrate level preincubated ... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50210772 (CHEMBL3916022) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum butyrylcholinesterase in presence of varying butyrylthiocholine iodide substrate level preincubated for 20 min... | Eur J Med Chem 125: 430-434 (2017) Article DOI: 10.1016/j.ejmech.2016.09.051 BindingDB Entry DOI: 10.7270/Q2RJ4MG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 89 total ) | Next | Last >> |