

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013820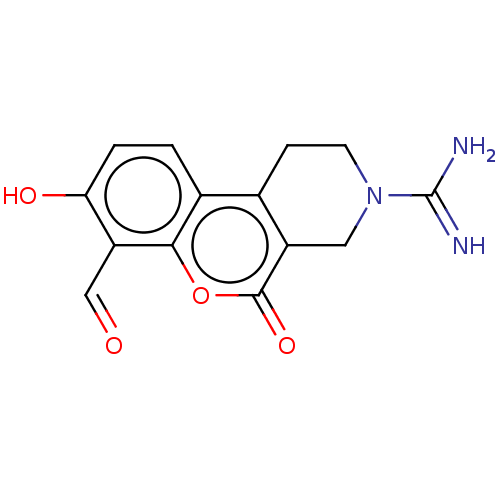 (CHEMBL3265274 | US10323013, Compound 34) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013802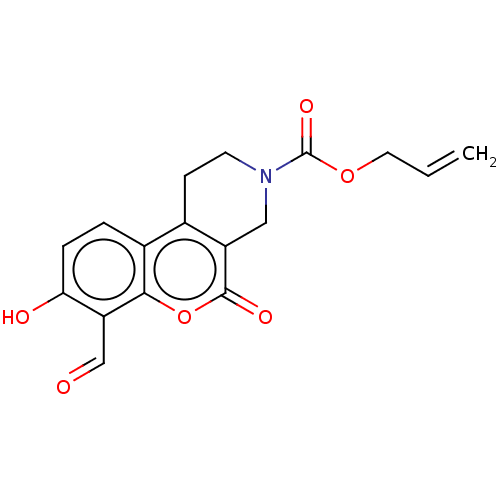 (CHEMBL3265261 | US10323013, Compound 21b) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013817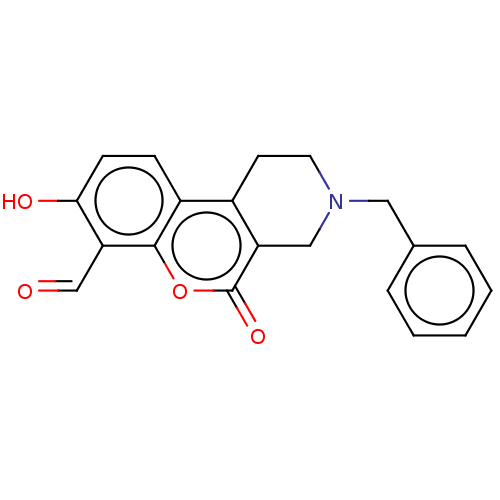 (CHEMBL3265271 | US10323013, Compound 31) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013814 (CHEMBL3265270 | US10323013, Compound 30) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013819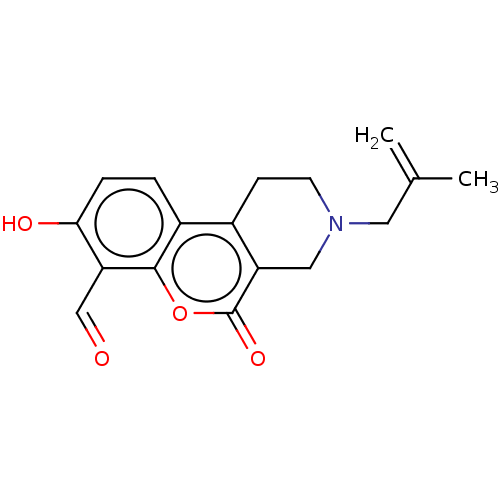 (CHEMBL3265273 | US10323013, Compound 33) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013818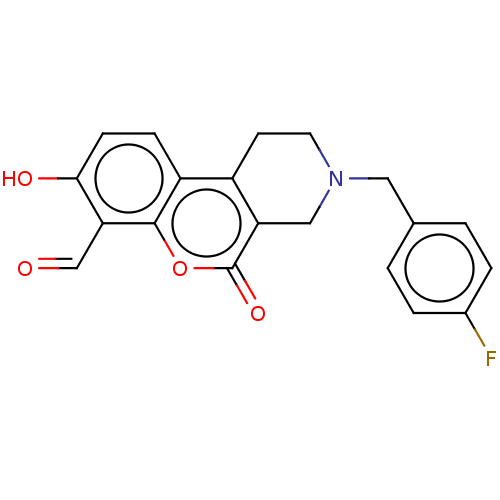 (CHEMBL3265272 | US10323013, Compound 32) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013813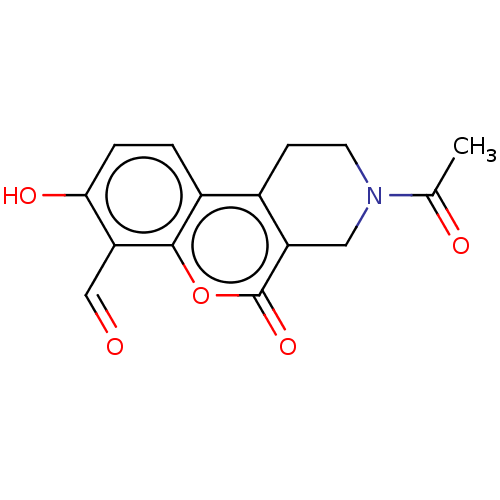 (CHEMBL3265269 | US10323013, Compound 29) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 312 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013812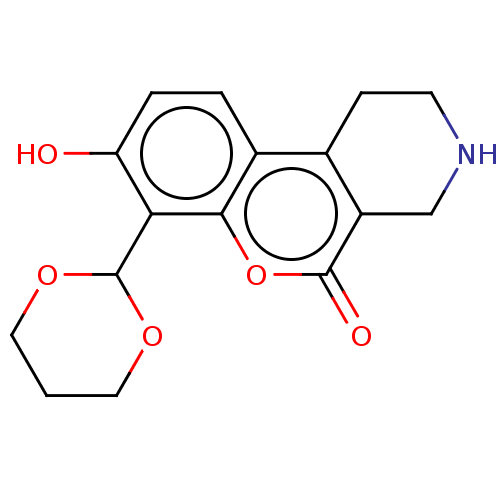 (CHEMBL3265268 | US10323013, Compound 28) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM416659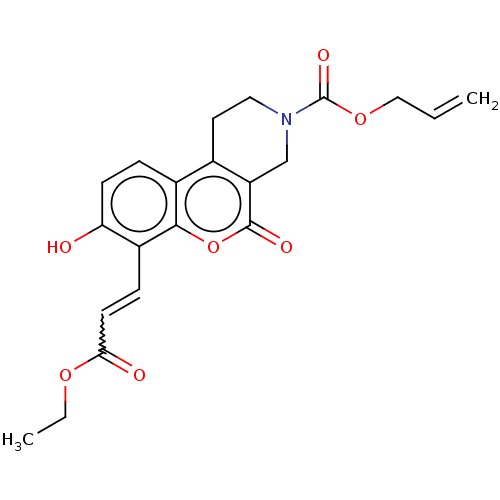 (US10323013, Compound 37 | allyl 7-(3-ethoxy-3-oxop...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013807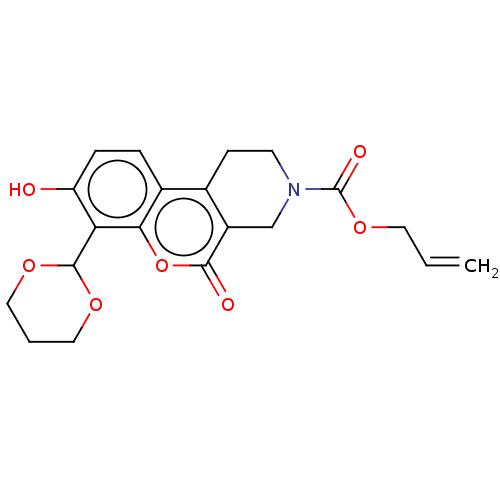 (CHEMBL3265264 | US10323013, Compound 24) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013825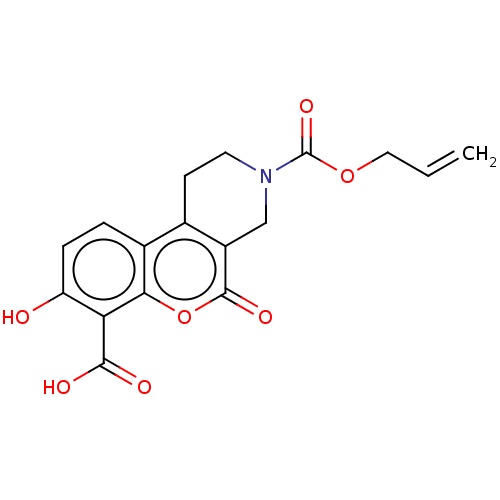 (CHEMBL3265279 | US10323013, Compound 40) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM416662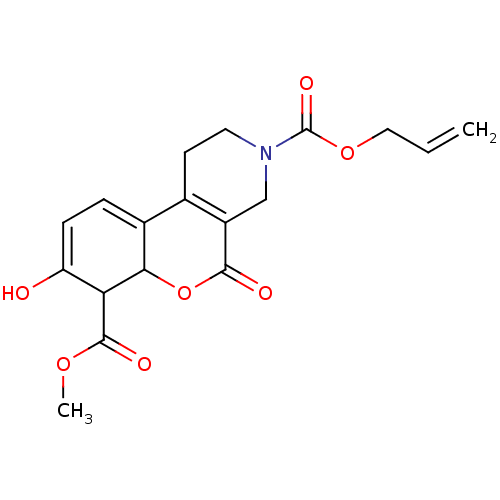 (3-allyl 7-methyl 8-hydroxy-5-oxo-4,5-dihydro-1H-ch...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013808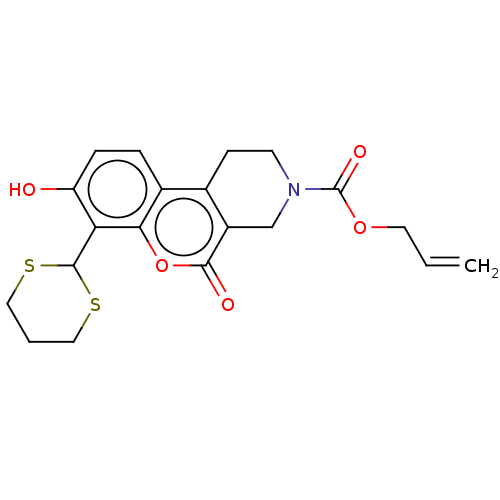 (CHEMBL3265265 | US10323013, Compound 25) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013821 (CHEMBL3265275 | US10323013, Compound 35) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013822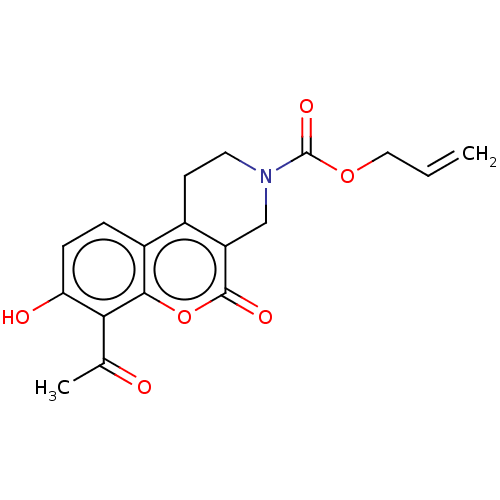 (CHEMBL3265276 | US10323013, Compound 36) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013810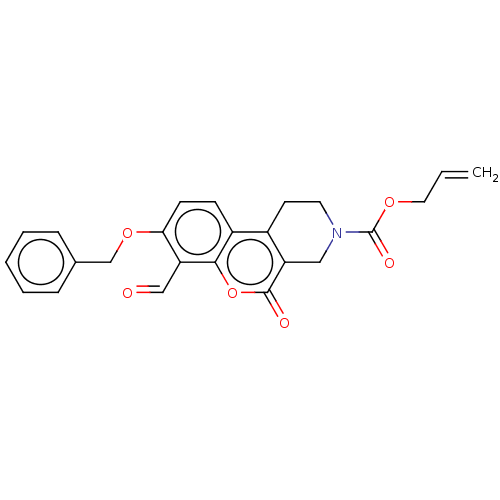 (CHEMBL3265267 | US10323013, Compound 27) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM416660 (US10323013, Compound 38 | allyl 8-hydroxy-7-(2-(me...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013803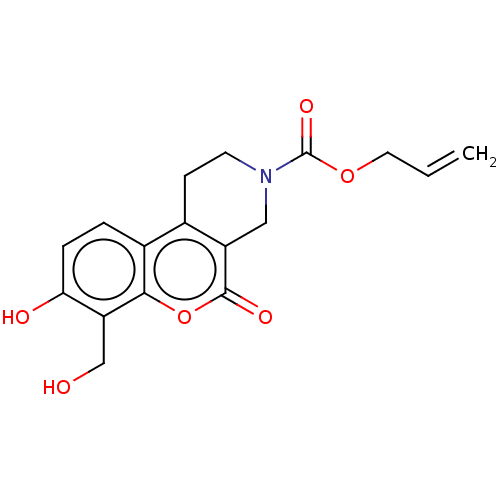 (CHEMBL3265262 | US10323013, Compound 22) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013827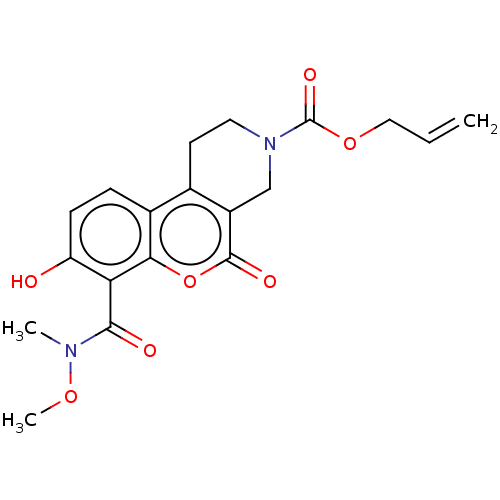 (CHEMBL3265281 | US10323013, Compound 42) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM50013809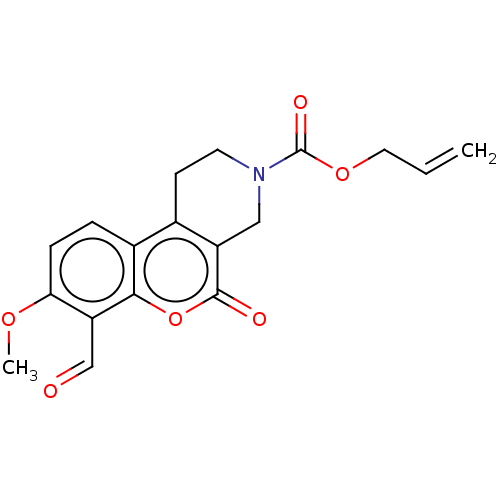 (CHEMBL3265266 | US10323013, Compound 26) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase/endoribonuclease IRE1 (Homo sapiens (Human)) | BDBM416640 (US10323013, Compound 23 | allyl 8-hydroxy-7-chloro...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
H. LEE MOFFITT CANCER CENTER AND RESEARCH INSTITUTE, INC. US Patent | Assay Description All compounds were evaluated by FRET-suppression assay in side-by-side experiments using 21b as a control inhibitor (Table 2). Protection of the alde... | US Patent US10323013 (2019) BindingDB Entry DOI: 10.7270/Q24J0HH9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||